Small molecule targets Env for endoplasmic reticulum-associated protein degradation and inhibits human immunodeficiency virus type 1 propagation
- PMID: 19640982
- PMCID: PMC2748041
- DOI: 10.1128/JVI.01700-08
Small molecule targets Env for endoplasmic reticulum-associated protein degradation and inhibits human immunodeficiency virus type 1 propagation
Abstract
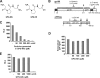
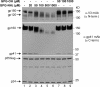
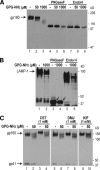
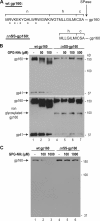
Human immunodeficiency virus type 1 (HIV-1) is dependent on its envelope glycoprotein (Env) to bind, fuse, and subsequently infect a cell. We show here that treatment of HIV-1-infected cells with glycyl-prolyl-glycine amide (GPG-NH(2)), dramatically reduced the infectivity of the released viral particles by decreasing their Env incorporation. The mechanism of GPG-NH(2) was uncovered by examining Env expression and maturation in treated cells. GPG-NH(2) treatment was found to affect Env by significantly decreasing its steady-state levels, its processing into gp120/gp41, and its mass by inducing glycan removal in a manner dependent on its native signal sequence and the proteasome. Therefore, GPG-NH(2) negatively impacts Env maturation, facilitating its targeting for endoplasmic reticulum-associated protein degradation, where Env is deglycosylated en route to its degradation. These findings illustrate that nontoxic drugs such as GPG-NH(2), which can selectively target glycoproteins to existing cellular degradation pathways, may be useful for pathogen therapy.
Figures

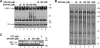

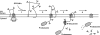
Similar articles
-
GPG-NH2 acts via the metabolite alphaHGA to target HIV-1 Env to the ER-associated protein degradation pathway.Retrovirology. 2010 Mar 15;7:20. doi: 10.1186/1742-4690-7-20. Retrovirology. 2010. PMID: 20230608 Free PMC article.
-
The tripeptide glycyl-prolyl-glycine amide does not affect the early steps of the human immunodeficiency virus type 1 replication.J Hum Virol. 2001 Jan-Feb;4(1):8-15. J Hum Virol. 2001. PMID: 11213934
-
Retention of the human immunodeficiency virus type 1 envelope glycoprotein in the endoplasmic reticulum does not redirect virus assembly from the plasma membrane.J Virol. 1998 Sep;72(9):7523-31. doi: 10.1128/JVI.72.9.7523-7531.1998. J Virol. 1998. PMID: 9696849 Free PMC article.
-
The mitochondrial translocator protein, TSPO, inhibits HIV-1 envelope glycoprotein biosynthesis via the endoplasmic reticulum-associated protein degradation pathway.J Virol. 2014 Mar;88(6):3474-84. doi: 10.1128/JVI.03286-13. Epub 2014 Jan 8. J Virol. 2014. PMID: 24403586 Free PMC article.
-
HIV-1 replication.Somat Cell Mol Genet. 2001 Nov;26(1-6):13-33. doi: 10.1023/a:1021070512287. Somat Cell Mol Genet. 2001. PMID: 12465460 Review.
Cited by
-
Type II transmembrane domain hydrophobicity dictates the cotranslational dependence for inversion.Mol Biol Cell. 2014 Nov 1;25(21):3363-74. doi: 10.1091/mbc.E14-04-0874. Epub 2014 Aug 27. Mol Biol Cell. 2014. PMID: 25165139 Free PMC article.
-
The Botanical Glycoside Oleandrin Inhibits Human T-cell Leukemia Virus Type-1 Infectivity and Env-Dependent Virological Synapse Formation.J Antivir Antiretrovir. 2019;11(3):184. doi: 10.35248/1948-5964.19.11.184. Epub 2019 Aug 21. J Antivir Antiretrovir. 2019. PMID: 31824586 Free PMC article.
-
HIV-1 Envelope Glycoprotein at the Interface of Host Restriction and Virus Evasion.Viruses. 2019 Mar 30;11(4):311. doi: 10.3390/v11040311. Viruses. 2019. PMID: 30935048 Free PMC article. Review.
-
Human Ubc9 is involved in intracellular HIV-1 Env stability after trafficking out of the trans-Golgi network in a Gag dependent manner.PLoS One. 2013 Jul 8;8(7):e69359. doi: 10.1371/journal.pone.0069359. Print 2013. PLoS One. 2013. PMID: 23861967 Free PMC article.
-
A novel piperazine derivative that targets hepatitis B surface antigen effectively inhibits tenofovir resistant hepatitis B virus.Sci Rep. 2021 Jun 3;11(1):11723. doi: 10.1038/s41598-021-91196-1. Sci Rep. 2021. PMID: 34083665 Free PMC article.
References
-
- Abacioglu, Y. H., T. R. Fouts, J. D. Laman, E. Claassen, S. H. Pincus, J. P. Moore, C. A. Roby, R. Kamin-Lewis, and G. K. Lewis. 1994. Epitope mapping and topology of baculovirus-expressed HIV-1 gp160 determined with a panel of murine monoclonal antibodies. AIDS Res. Hum. Retrovir. 10:371-381. - PubMed
-
- Ahner, A., and J. L. Brodsky. 2004. Checkpoints in ER-associated degradation: excuse me, which way to the proteasome? Trends Cell Biol. 14:474-478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

