Small molecule targets Env for endoplasmic reticulum-associated protein degradation and inhibits human immunodeficiency virus type 1 propagation
- PMID: 19640982
- PMCID: PMC2748041
- DOI: 10.1128/JVI.01700-08
Small molecule targets Env for endoplasmic reticulum-associated protein degradation and inhibits human immunodeficiency virus type 1 propagation
Abstract
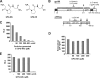
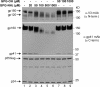
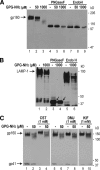
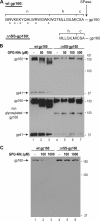
Human immunodeficiency virus type 1 (HIV-1) is dependent on its envelope glycoprotein (Env) to bind, fuse, and subsequently infect a cell. We show here that treatment of HIV-1-infected cells with glycyl-prolyl-glycine amide (GPG-NH(2)), dramatically reduced the infectivity of the released viral particles by decreasing their Env incorporation. The mechanism of GPG-NH(2) was uncovered by examining Env expression and maturation in treated cells. GPG-NH(2) treatment was found to affect Env by significantly decreasing its steady-state levels, its processing into gp120/gp41, and its mass by inducing glycan removal in a manner dependent on its native signal sequence and the proteasome. Therefore, GPG-NH(2) negatively impacts Env maturation, facilitating its targeting for endoplasmic reticulum-associated protein degradation, where Env is deglycosylated en route to its degradation. These findings illustrate that nontoxic drugs such as GPG-NH(2), which can selectively target glycoproteins to existing cellular degradation pathways, may be useful for pathogen therapy.
Figures

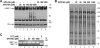

References
-
- Abacioglu, Y. H., T. R. Fouts, J. D. Laman, E. Claassen, S. H. Pincus, J. P. Moore, C. A. Roby, R. Kamin-Lewis, and G. K. Lewis. 1994. Epitope mapping and topology of baculovirus-expressed HIV-1 gp160 determined with a panel of murine monoclonal antibodies. AIDS Res. Hum. Retrovir. 10:371-381. - PubMed
-
- Ahner, A., and J. L. Brodsky. 2004. Checkpoints in ER-associated degradation: excuse me, which way to the proteasome? Trends Cell Biol. 14:474-478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

