Latency-associated transcript (LAT) exon 1 controls herpes simplex virus species-specific phenotypes: reactivation in the guinea pig genital model and neuron subtype-specific latent expression of LAT
- PMID: 19641003
- PMCID: PMC2748022
- DOI: 10.1128/JVI.00559-09
Latency-associated transcript (LAT) exon 1 controls herpes simplex virus species-specific phenotypes: reactivation in the guinea pig genital model and neuron subtype-specific latent expression of LAT
Abstract
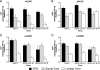
Herpes simplex virus 1 (HSV-1) and HSV-2 cause similar acute infections but differ in their abilities to reactivate from trigeminal and lumbosacral dorsal root ganglia. During latency, HSV-1 and HSV-2 also preferentially express their latency-associated transcripts (LATs) in different sensory neuronal subtypes that are positive for A5 and KH10 markers, respectively. Chimeric virus studies showed that LAT region sequences influence both of these viral species-specific phenotypes. To further map the LAT region sequences responsible for these phenotypes, we constructed the chimeric virus HSV2-LAT-E1, in which exon 1 (from the LAT TATA to the intron splice site) was replaced by the corresponding sequence from HSV-1 LAT. In intravaginally infected guinea pigs, HSV2-LAT-E1 reactivated inefficiently relative to the efficiency of its rescuant and wild-type HSV-2, but it yielded similar levels of viral DNA, LAT, and ICP0 during acute and latent infection. HSV2-LAT-E1 preferentially expressed the LAT in A5+ neurons (as does HSV-1), while the chimeric viruses HSV2-LAT-P1 (LAT promoter swap) and HSV2-LAT-S1 (LAT sequence swap downstream of the promoter) exhibited neuron subtype-specific latent LAT expression phenotypes more similar to that of HSV-2 than that of HSV-1. Rescuant viruses displayed the wild-type HSV-2 phenotypes of efficient reactivation in the guinea pig genital model and a tendency to express LAT in KH10+ neurons. The region that is critical for HSV species-specific differences in latency and reactivation thus lies between the LAT TATA and the intron splice site, and minor differences in the 5' ends of chimeric sequences in HSV2-LAT-E1 and HSV2-LAT-S1 point to sequences immediately downstream of the LAT TATA.
Figures




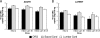

References
-
- Batchelor, A. H., K. W. Wilcox, and P. O'Hare. 1994. Binding and repression of the latency-associated promoter of herpes simplex virus by the immediate early 175K protein. J. Gen. Virol. 75:753-767. - PubMed
-
- Bhattacharjee, P. S., R. K. Tran, M. E. Myles, K. Maruyama, A. Mallakin, D. C. Bloom, and J. M. Hill. 2003. Overlapping subdeletions within a 348-bp in the 5′ exon of the LAT region that facilitates epinephrine-induced reactivation of HSV-1 in the rabbit ocular model do not further define a functional element. Virology 312:151-158. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

