Interactions between auxin and strigolactone in shoot branching control
- PMID: 19641034
- PMCID: PMC2735998
- DOI: 10.1104/pp.109.137646
Interactions between auxin and strigolactone in shoot branching control
Abstract
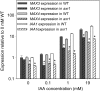
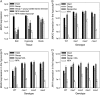
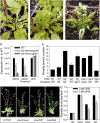
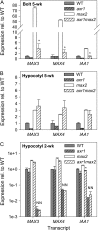
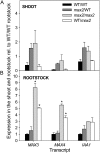
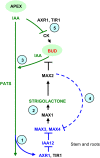
In Arabidopsis (Arabidopsis thaliana), the carotenoid cleavage dioxygenases MORE AXILLARY GROWTH3 (MAX3) and MAX4 act together with MAX1 to produce a strigolactone signaling molecule required for the inhibition of axillary bud outgrowth. We show that both MAX3 and MAX4 transcripts are positively auxin regulated in a manner similar to the orthologous genes from pea (Pisum sativum) and rice (Oryza sativa), supporting evolutionary conservation of this regulation in plants. This regulation is important for branching control because large auxin-related reductions in these transcripts are associated with increased axillary branching. Both transcripts are up-regulated in max mutants, and consistent with max mutants having increased auxin in the polar auxin transport stream, this feedback regulation involves auxin signaling. We suggest that both auxin and strigolactone have the capacity to modulate each other's levels and distribution in a dynamic feedback loop required for the coordinated control of axillary branching.
Figures
Similar articles
-
The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport.Curr Biol. 2006 Mar 21;16(6):553-63. doi: 10.1016/j.cub.2006.01.058. Curr Biol. 2006. PMID: 16546078
-
Connective auxin transport contributes to strigolactone-mediated shoot branching control independent of the transcription factor BRC1.PLoS Genet. 2019 Mar 13;15(3):e1008023. doi: 10.1371/journal.pgen.1008023. eCollection 2019 Mar. PLoS Genet. 2019. PMID: 30865619 Free PMC article.
-
The Arabidopsis ortholog of rice DWARF27 acts upstream of MAX1 in the control of plant development by strigolactones.Plant Physiol. 2012 Jul;159(3):1073-85. doi: 10.1104/pp.112.196253. Epub 2012 May 22. Plant Physiol. 2012. PMID: 22623516 Free PMC article.
-
New genes in the strigolactone-related shoot branching pathway.Curr Opin Plant Biol. 2010 Feb;13(1):34-9. doi: 10.1016/j.pbi.2009.10.003. Epub 2009 Nov 11. Curr Opin Plant Biol. 2010. PMID: 19913454 Review.
-
Strigolactones: discovery of the elusive shoot branching hormone.Trends Plant Sci. 2009 Jul;14(7):364-72. doi: 10.1016/j.tplants.2009.04.003. Epub 2009 Jun 17. Trends Plant Sci. 2009. PMID: 19540149 Review.
Cited by
-
Roles of DgBRC1 in regulation of lateral branching in chrysanthemum (Dendranthema ×grandiflora cv. Jinba).PLoS One. 2013 Apr 17;8(4):e61717. doi: 10.1371/journal.pone.0061717. Print 2013. PLoS One. 2013. PMID: 23613914 Free PMC article.
-
The importance of strigolactone transport regulation for symbiotic signaling and shoot branching.Planta. 2016 Jun;243(6):1351-60. doi: 10.1007/s00425-016-2503-9. Epub 2016 Apr 4. Planta. 2016. PMID: 27040840 Free PMC article. Review.
-
A study of CCD8 genes/proteins in seven monocots and eight dicots.PLoS One. 2019 Mar 12;14(3):e0213531. doi: 10.1371/journal.pone.0213531. eCollection 2019. PLoS One. 2019. PMID: 30861026 Free PMC article.
-
Harmony but Not Uniformity: Role of Strigolactone in Plants.Biomolecules. 2021 Nov 1;11(11):1616. doi: 10.3390/biom11111616. Biomolecules. 2021. PMID: 34827614 Free PMC article. Review.
-
Genetic insights into the regulatory pathways for continuous flowering in a unique orchid Arundina graminifolia.BMC Plant Biol. 2021 Dec 10;21(1):587. doi: 10.1186/s12870-021-03350-6. BMC Plant Biol. 2021. PMID: 34893019 Free PMC article.
References
-
- Abel S, Nguyen MD, Theologis A (1995) The PS-IAA4/5-like family of early auxin-inducible mRNAs in Arabidopsis thaliana. J Mol Biol 251 533–549 - PubMed
-
- Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435 824–827 - PubMed
-
- Arite T, Iwata H, Ohshima K, Maekawa M, Nakajima M, Kojima M, Sakakibara H, Kyozuka J (2007) DWARF10, an RMS1/MAX4/DAD1 ortholog, controls lateral bud outgrowth in rice. Plant J 51 1019–1029 - PubMed
-
- Auldridge ME, Block A, Vogel JT, Dabney-Smith C, Mila I, Bouzayen M, Magallanes-Lundback M, DellaPenna D, McCarty DR, Klee HJ (2006) Characterisation of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant J 45 982–993 - PubMed
-
- Bainbridge K, Sorefan K, Ward S, Leyser O (2005) Hormonally controlled expression of the Arabidopsis MAX4 shoot branching regulatory gene. Plant J 44 569–580 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

