A rat excised larynx model of vocal fold scar
- PMID: 19641079
- PMCID: PMC2719832
- DOI: 10.1044/1092-4388(2009/08-0049)
A rat excised larynx model of vocal fold scar
Abstract
Purpose: To develop and evaluate a rat excised larynx model for the measurement of acoustic, aerodynamic, and vocal fold vibratory changes resulting from vocal fold scar.
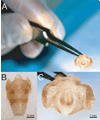
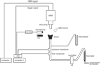
Method: Twenty-four 4-month-old male Sprague-Dawley rats were assigned to 1 of 4 experimental groups: chronic vocal fold scar, chronic vocal fold scar treated with 100-ng basic fibroblast growth factor (bFGF), chronic vocal fold scar treated with saline (sham treatment), and unscarred untreated control. Following tissue harvest, histological and immunohistochemical data were collected to confirm extracellular matrix alteration in the chronic scar group; acoustic, aerodynamic, and high-speed digital imaging data were collected using an excised larynx setup in all groups. Phonation threshold pressure (P(th)), glottal resistance (R(g)), glottal efficiency (E(g)), vibratory amplitude, and vibratory area were used as dependent variables.
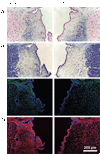
Results: Chronically scarred vocal folds were characterized by elevated collagen Types I and III and reduced hyaluronic acid abundance. Phonation was achieved, and data were collected from all control and bFGF-treated larynges; however, phonation was not achieved with 3 of 6 chronically scarred and 1 of 6 saline-treated larynges. Compared with control, the chronic scar group was characterized by elevated P(th), reduced E(g), and intralarynx vibratory amplitude and area asymmetry. The bFGF group was characterized by P(th) below control-group levels, E(g) comparable with control, and vocal fold vibratory amplitude and area symmetry comparable with control. The sham group was characterized by P(th) comparable with control, E(g) superior to control, and vocal fold vibratory amplitude and area symmetry comparable with control.
Conclusions: The excised larynx model reported here demonstrated robust deterioration across phonatory indices under the scar condition and sensitivity to treatment-induced change under the bFGF condition. The improvement observed under the sham condition may reflect unanticipated therapeutic benefit or artifact. This model holds promise as a tool for the functional characterization of biomechanical tissue changes resulting from vocal fold scar and the evaluation of experimental therapies.
Figures




References
-
- Akita S, Akino K, Tanaka K, Anraku K, Hirano A. A basic fibroblast growth factor improves lower extremity wound healing with a porcine-derived skin substitute. Journal of Trauma: Injury, Infection and Critical Care. 2008;64:809–815. - PubMed
-
- Alipour F, Jaiswal S. Glottal airflow resistance in excised pig, sheep, and cow larynges. Journal of Voice. in press. - PubMed
-
- Alipour F, Jaiswal S, Finnegan E. Aerodynamic and acoustic effects of false vocal folds and epiglottis in excised larynx models. Annals of Otology, Rhinology and Laryngology. 2007;116:135–144. - PubMed
-
- Alipour F, Scherer RC. On pressure-frequency relations in the excised larynx. Journal of the Acoustical Society of America. 2007;122:2296–2305. - PubMed
-
- Benninger MS, Alessi D, Archer S, Bastian R, Ford CN, Koufman J, et al. Vocal fold scarring: Current concepts and management. Otolaryngology—Head and Neck Surgery. 1996;115:474–482. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

