Dynein light chain LC8 regulates syntaphilin-mediated mitochondrial docking in axons
- PMID: 19641106
- PMCID: PMC6666546
- DOI: 10.1523/JNEUROSCI.1472-09.2009
Dynein light chain LC8 regulates syntaphilin-mediated mitochondrial docking in axons
Abstract
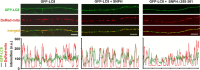
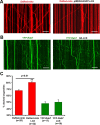
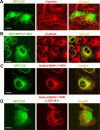
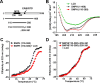
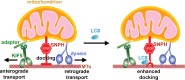
Mitochondria in the cell bodies of neurons are transported down neuronal processes in response to changes in local energy and metabolic states. Because of their extreme polarity, neurons require specialized mechanisms to regulate mitochondrial transport and retention in axons. Our previous studies using syntaphilin (snph) knock-out mice provided evidence that SNPH targets to axonal mitochondria and controls their mobility through its static interaction with microtubules (MTs). However, the mechanisms regulating SNPH-mediated mitochondrial docking remain elusive. Here, we report an unexpected role for dynein light chain LC8. Using proteomic biochemical and cell biological assays combined with time-lapse imaging in live snph wild-type and mutant neurons, we reveal that LC8 regulates axonal mitochondrial mobility by binding to SNPH, thus enhancing the SNPH-MT docking interaction. Using mutagenesis assays, we mapped a seven-residue LC8-binding motif. Through this specific interaction, SNPH recruits LC8 to axonal mitochondria; such colocalization is abolished when neurons express SNPH mutants lacking the LC8-binding motif. Transient LC8 expression reduces mitochondrial mobility in snph (+/+) but not (-/-) neurons, suggesting that the observed effect of LC8 depends on the SNPH-mediated docking mechanism. In contrast, deleting the LC8-binding motif impairs the ability of SNPH to immobilize axonal mitochondria. Furthermore, circular dichroism spectrum analysis shows that LC8 stabilizes an alpha-helical coiled-coil within the MT-binding domain of SNPH against thermal unfolding. Thus, our study provides new mechanistic insights into controlling mitochondrial mobility through a dynamic interaction between the mitochondrial docking receptor and axonal cytoskeleton.
Figures




References
-
- Barbar E. Dynein light chain LC8 is a dimerization hub essential in diverse protein networks. Biochemistry. 2008;47:503–508. - PubMed
-
- Barbar E, Kleinman B, Imhoff D, Li M, Hays TS, Hare M. Dimerization and folding of LC8, a highly conserved light chain of cytoplasmic dynein. Biochemistry. 2001;40:1596–1605. - PubMed
-
- Benashski SE, Harrison A, Patel-King RS, King SM. Dimerization of the highly conserved light chain shared by dynein and myosin V. J Biol Chem. 1997;272:20929–20935. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
