Deviant ryanodine receptor-mediated calcium release resets synaptic homeostasis in presymptomatic 3xTg-AD mice
- PMID: 19641109
- PMCID: PMC6666542
- DOI: 10.1523/JNEUROSCI.2047-09.2009
Deviant ryanodine receptor-mediated calcium release resets synaptic homeostasis in presymptomatic 3xTg-AD mice
Abstract
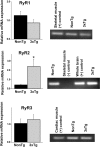
Presenilin mutations result in exaggerated endoplasmic reticulum (ER) calcium release in cellular and animal models of Alzheimer's disease (AD). In this study, we examined whether dysregulated ER calcium release in young 3xTg-AD neurons alters synaptic transmission and plasticity mechanisms before the onset of histopathology and cognitive deficits. Using electrophysiological recordings and two-photon calcium imaging in young (6-8 weeks old) 3xTg-AD and non-transgenic (NonTg) hippocampal slices, we show a marked increase in ryanodine receptor (RyR)-evoked calcium release within synapse-dense regions of CA1 pyramidal neurons. In addition, we uncovered a deviant contribution of presynaptic and postsynaptic ryanodine receptor-sensitive calcium stores to synaptic transmission and plasticity in 3xTg-AD mice that is not present in NonTg mice. As a possible underlying mechanism, the RyR2 isoform was found to be selectively increased more than fivefold in the hippocampus of 3xTg-AD mice relative to the NonTg controls. These novel findings demonstrate that 3xTg-AD CA1 neurons at presymptomatic ages operate under an aberrant, yet seemingly functional, calcium signaling and synaptic transmission system long before AD histopathology onset. These early signaling alterations may underlie the later synaptic breakdown and cognitive deficits characteristic of later stage AD.
Figures








References
-
- Bardo S, Cavazzini MG, Emptage N. The role of endoplasmic reticulum calcium stores in the plasticity of central neurons. Trends Pharmacol Sci. 2006;27:78–84. - PubMed
-
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. - PubMed
-
- Bliss TV, Collingridge GL. A synaptic model of memory: LTP in the hippocampus. Nature. 1993;361:31–39. - PubMed
-
- Bouchard R, Pattarini R, Geiger JD. Presence and functional significance of presynaptic ryanodine receptors. Prog Neurobiol. 2003;69:391–418. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
