A microsaccadic rhythm modulates gamma-band synchronization and behavior
- PMID: 19641110
- PMCID: PMC6666524
- DOI: 10.1523/JNEUROSCI.1193-09.2009
A microsaccadic rhythm modulates gamma-band synchronization and behavior
Abstract
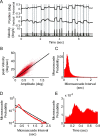
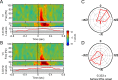
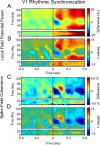
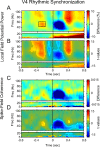
Rhythms occur both in neuronal activity and in behavior. Behavioral rhythms abound at frequencies at or below 10 Hz. Neuronal rhythms cover a very wide frequency range, and the phase of neuronal low-frequency rhythms often rhythmically modulates the strength of higher-frequency rhythms, particularly of gamma-band synchronization (GBS). Here, we study stimulus-induced GBS in awake monkey areas V1 and V4 in relation to a specific form of spontaneous behavior, namely microsaccades (MSs), small fixational eye movements. We found that MSs occur rhythmically at a frequency of approximately 3.3 Hz. The rhythmic MSs were predicted by the phase of the 3.3 Hz rhythm in V1 and V4 local field potentials. In turn, the MSs modulated both visually induced GBS and the speed of visually triggered behavioral responses. Fast/slow responses were preceded by a specific temporal pattern of MSs. These MS patterns induced perturbations in GBS that in turn explained variability in behavioral response speed. We hypothesize that the 3.3 Hz rhythm structures the sampling and exploration of the environment through building and breaking neuronal ensembles synchronized in the gamma-frequency band to process sensory stimuli.
Figures




References
-
- Ahissar E, Arieli A. Figuring space by time. Neuron. 2001;32:185–201. - PubMed
-
- Bair W, O'Keefe LP. The influence of fixational eye movements on the response of neurons in area MT of the macaque. Vis Neurosci. 1998;15:779–786. - PubMed
-
- Beeler GW., Jr Visual threshold changes resulting from spontaneous saccadic eye movements. Vision Res. 1967;7:769–775. - PubMed
-
- Bichot NP, Rossi AF, Desimone R. Parallel and serial neural mechanisms for visual search in macaque area V4. Science. 2005;308:529–534. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
