Mesencephalic astrocyte-derived neurotrophic factor is neurorestorative in rat model of Parkinson's disease
- PMID: 19641128
- PMCID: PMC6666534
- DOI: 10.1523/JNEUROSCI.0833-09.2009
Mesencephalic astrocyte-derived neurotrophic factor is neurorestorative in rat model of Parkinson's disease
Abstract
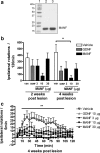
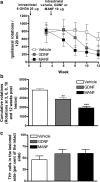
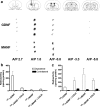
Neurotrophic factors are promising candidates for the treatment of Parkinson's disease (PD). Mesencephalic astrocyte-derived neurotrophic factor (MANF) belongs to a novel evolutionarily conserved family of neurotrophic factors. We examined whether MANF has neuroprotective and neurorestorative effect in an experimental model of PD in rats. We also studied the distribution and transportation of intrastriatally injected MANF in the brain and compared it with glial cell line-derived neurotrophic factor (GDNF). Unilateral lesion of nigrostriatal dopaminergic system was induced by intrastriatal injection of 6-hydroxydopamine (6-OHDA). Amphetamine-induced turning behavior was monitored up to 12 weeks after the unilateral lesion. The local diffusion at the injection site and transportation profiles of intrastriatally injected MANF and GDNF were studied by immunohistochemical detection of the unlabeled growth factors as well as by autoradiographic and gamma counting detection of (125)I-labeled trophic factors. Intrastriatally injected MANF protected nigrostriatal dopaminergic nerves from 6-OHDA-induced degeneration as evaluated by counting tyrosine hydroxylase (TH)-positive cell bodies in the substantia nigra (SN) and TH-positive fibers in the striatum. More importantly, MANF also restored the function of the nigrostriatal dopaminergic system when administered either 6 h before or 4 weeks after 6-OHDA administration in the striatum. MANF was distributed throughout the striatum more readily than GDNF. The mechanism of MANF action differs from that of GDNF because intrastriatally injected (125)I-MANF was transported to the frontal cortex, whereas (125)I-GDNF was transported to the SN. Our results suggest that MANF is readily distributed throughout the striatum and has significant therapeutic potential for the treatment of PD.
Figures



Similar articles
-
Comparative study of the neurotrophic effects elicited by VEGF-B and GDNF in preclinical in vivo models of Parkinson's disease.Neuroscience. 2014 Jan 31;258:385-400. doi: 10.1016/j.neuroscience.2013.11.038. Epub 2013 Nov 27. Neuroscience. 2014. PMID: 24291725 Free PMC article.
-
Chronic infusion of CDNF prevents 6-OHDA-induced deficits in a rat model of Parkinson's disease.Exp Neurol. 2011 Mar;228(1):99-108. doi: 10.1016/j.expneurol.2010.12.013. Epub 2010 Dec 24. Exp Neurol. 2011. PMID: 21185834
-
Glial cell line-derived neurotrophic factor (GDNF) gene delivery protects dopaminergic terminals from degeneration.Exp Neurol. 2001 May;169(1):83-95. doi: 10.1006/exnr.2001.7638. Exp Neurol. 2001. PMID: 11312561
-
Towards a neuroprotective gene therapy for Parkinson's disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model.Brain Res. 2000 Dec 15;886(1-2):82-98. doi: 10.1016/s0006-8993(00)02915-2. Brain Res. 2000. PMID: 11119690 Review.
-
Adenoviral vector-mediated delivery of glial cell line-derived neurotrophic factor provides neuroprotection in the aged parkinsonian rat.Clin Exp Pharmacol Physiol. 2001 Nov;28(11):896-900. doi: 10.1046/j.1440-1681.2001.03544.x. Clin Exp Pharmacol Physiol. 2001. PMID: 11703392 Review.
Cited by
-
Mesencephalic Astrocyte-Derived Neurotrophic Factor (MANF) Regulates Neurite Outgrowth Through the Activation of Akt/mTOR and Erk/mTOR Signaling Pathways.Front Mol Neurosci. 2020 Sep 24;13:560020. doi: 10.3389/fnmol.2020.560020. eCollection 2020. Front Mol Neurosci. 2020. PMID: 33071755 Free PMC article.
-
Mesencephalic astrocyte-derived neurotrophic factor (MANF) protects against Aβ toxicity via attenuating Aβ-induced endoplasmic reticulum stress.J Neuroinflammation. 2019 Feb 13;16(1):35. doi: 10.1186/s12974-019-1429-0. J Neuroinflammation. 2019. PMID: 30760285 Free PMC article.
-
Cerebral Dopamine Neurotrophic Factor (CDNF) Has Neuroprotective Effects against Cerebral Ischemia That May Occur through the Endoplasmic Reticulum Stress Pathway.Int J Mol Sci. 2018 Jun 29;19(7):1905. doi: 10.3390/ijms19071905. Int J Mol Sci. 2018. PMID: 29966219 Free PMC article.
-
Expression and Distribution of Mesencephalic Astrocyte-Derived Neurotrophic Factor in the Retina and Optic Nerve.Front Hum Neurosci. 2017 Jan 19;10:686. doi: 10.3389/fnhum.2016.00686. eCollection 2016. Front Hum Neurosci. 2017. PMID: 28154531 Free PMC article.
-
Mesencephalic astrocyte-derived neurotrophic factor protects the heart from ischemic damage and is selectively secreted upon sarco/endoplasmic reticulum calcium depletion.J Biol Chem. 2012 Jul 27;287(31):25893-904. doi: 10.1074/jbc.M112.356345. Epub 2012 May 25. J Biol Chem. 2012. PMID: 22637475 Free PMC article.
References
-
- Anderson KD, Alderson RF, Altar CA, DiStefano PS, Corcoran TL, Lindsay RM, Wiegand SJ. Differential distribution of exogenous BDNF, NGF, and NT-3 in the brain corresponds to the relative abundance and distribution of high-affinity and low-affinity neurotrophin receptors. J Comp Neurol. 1995;357:296–317. - PubMed
-
- Bespalov MM, Saarma M. GDNF family receptor complexes are emerging drug targets. Trends Pharmacol Sci. 2007;28:68–74. - PubMed
-
- Björklund A, Rosenblad C, Winkler C, Kirik D. Studies on neuroprotective and regenerative effects of GDNF in a partial lesion model of Parkinson's disease. Neurobiol Dis. 1997;4:186–200. - PubMed
-
- Cadet JL, Zhu SM. The intrastriatal 6-hydroxydopamine model of hemiparkinsonism: quantitative receptor autoradiographic evidence of correlation between circling behavior and presynaptic as well as postsynaptic nigrostriatal markers in the rat. Brain Res. 1992;595:316–326. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
