Catechol O-methyltransferase pharmacogenomics: human liver genotype-phenotype correlation and proximal promoter studies
- PMID: 19641441
- PMCID: PMC3052748
- DOI: 10.1097/FPC.0b013e32832c15c6
Catechol O-methyltransferase pharmacogenomics: human liver genotype-phenotype correlation and proximal promoter studies
Abstract
Objectives: Catechol O-methyltransferase (COMT) is expressed as both soluble (S) and membrane-bound (MB) isoforms, with S-COMT predominantly expressed in the liver. A common nonsynonymous single nucleotide polymorphism (SNP), 472G > A (108/158Val > Met, S/MB), has been associated with variation in levels of COMT enzyme activity and thermal stability. We set out to test the hypothesis that additional COMT polymorphisms might also be associated with phenotypic variation.
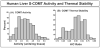
Methods: We phenotyped 268 liver biopsy samples for S-COMT activity and thermal stability, resequenced a portion of the gene that had not been resequenced earlier, and genotyped DNA from these same samples for 16 COMT polymorphisms.
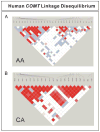
Results: There was a significant association between the two COMT phenotypes and genotype at the codon 108 SNP. A haplotype-based approach was then used to assess the possible association of other polymorphisms with phenotype. Specifically, the codon 108 SNP explained 20.4% of variance in enzyme activity (P < 10), and 59% of variance in thermal stability (P < 10). Haplotypes that included SNPs at cDNA nucleotides 408 and 472 explained additional variance in enzyme activity (up to 24.4%), and the addition to the haplotype of a SNP at intron 2 (51) explained a total of 27.5% of the variance. However, no SNPs beyond that at the nucleotide 472G > A polymorphism were associated with variation in thermal stability. We also observed a three-fold variation in the ability of reporter gene constructs for 'proximal promoter' haplotypes to drive transcription.
Conclusion: The common COMT 108Val > Met polymorphism is associated with human liver S-COMT activity and thermal stability, but additional COMT SNPs also contribute to variation in activity.
Figures




References
-
- Weinshilboum RM, Otterness DM, Szumlanski CL. Methylation pharmacogenetics: catechol O-methyltransferase, thiopurine methyltransferase, and histamine N-methyltransferase. Annu Rev Pharmacol Toxicol. 1999;39:19–52. - PubMed
-
- Tenhunen J, Salminen M, Lundstrom K, Kiviluoto T, Savolainen R, Ulmanen I. Genomic organization of the human catechol O-methyltransferase gene and its expression from two distinct promoters. Eur J Biochem. 1994;223:1049–59. - PubMed
-
- Lundström K, Tenhunen J, Tilgmann C, TK, Panula P, Ulmanen I. Cloning, expression and structure of catechol-O-methyltransferase. Biochim Biophys Acta. 1995;1251:1–10. - PubMed
-
- Jeffery DR, Roth JA. Characterization of membrane-bound and soluble catechol-O-mehyltransferase from human frontal cortex. J Neurochem. 1984;42:826–832. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

