Mimicking the BH3 domain to kill cancer cells
- PMID: 19641500
- PMCID: PMC3733265
- DOI: 10.1038/onc.2009.52
Mimicking the BH3 domain to kill cancer cells
Abstract
Cancer cells show deviant behavior that induces apoptotic signaling. To survive, cancer cells typically acquire changes enabling evasion of death signals. One way they do this is by increasing the expression of anti-apoptotic BCL-2 proteins. Anti-apoptotic BCL-2 family proteins antagonize death signaling by forming heterodimers with pro-death proteins. Heterodimer formation occurs through binding of the pro-apoptotic protein's BH3 domain into the hydrophobic cleft of anti-apoptotic proteins. The BH3 mimetics are small molecule antagonists of the anti-apoptotic BCL-2 members that function as competitive inhibitors by binding to the hydrophobic cleft. Under certain conditions, antagonism of anti-apoptotic BCL-2 family proteins can unleash pro-death molecules in cancer cells. Thus, the BH3 mimetics are a new class of cancer drugs that specifically target a mechanism of cancer cell survival to selectively kill cancer cells.
Figures


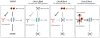

References
-
- Acehan D, Jiang X, Morgan DG, Heuser JE, Wang X, Akey CW. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol Cell. 2002;9:423–32. - PubMed
-
- Banin S, Moyal L, Shieh S, Taya Y, Anderson CW, Chessa L, et al. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–7. - PubMed
-
- Becattini B, Kitada S, Leone M, Monosov E, Chandler S, Zhai D, et al. Rational design and real time, in-cell detection of the proapoptotic activity of a novel compound targeting Bcl-X(L) Chem Biol. 2004;11:389–95. - PubMed
-
- Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, et al. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. - PubMed
-
- Boyd JM, Gallo GJ, Elangovan B, Houghton AB, Malstrom S, Avery BJ, et al. Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene. 1995;11:1921–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

