BH3-only proteins in apoptosis and beyond: an overview
- PMID: 19641503
- PMCID: PMC2928556
- DOI: 10.1038/onc.2009.39
BH3-only proteins in apoptosis and beyond: an overview
Abstract
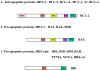
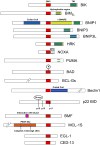
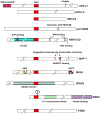
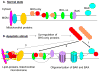
BH3-only BCL-2 family proteins are effectors of canonical mitochondrial apoptosis. They discharge their pro-apoptotic functions through BH1-3 pro-apoptotic proteins such as BAX and BAK, while their activity is suppressed by BH1-4 anti-apoptotic BCL-2 family members. The precise mechanism by which BH3-only proteins mediate apoptosis remains unresolved. The existing data are consistent with three mutually non-exclusive models (1) displacement of BH1-3 proteins from complexes with BH1-4 proteins; (2) direct interaction with and conformational activation of BH1-3 proteins; and (3) membrane insertion and membrane remodeling. The BH3-only proteins appear to play critical roles in restraining cancer and inflammatory diseases such as rheumatoid arthritis. Molecules that mimic the effect of BH3-only proteins are being used in treatments against these diseases. The cell death activity of a subclass of BH3-only members (BNIP3 and BNIP3L) is linked to cardiomyocyte loss during heart failure. In addition to their established role in apoptosis, several BH3-only members also regulate diverse cellular functions in cell-cycle regulation, DNA repair and metabolism. Several members are implicated in the induction of autophagy and autophagic cell death, possibly through unleashing of the BH3-only autophagic effector Beclin 1 from complexes with BCL-2/BCL-xL. The Chapters included in the current Oncogene Review issues provide in-depth discussions on various aspects of major BH3-only proteins.
Figures

References
-
- Arena V, Martini M, Luongo M, Capelli A, Larocca LM. Mutations of the BIK gene in human peripheral B-cell lymphomas. Genes Chromosomes Cancer. 2003;38:91–96. - PubMed
-
- Bachmann PS, Gorman R, Mackenzie KL, Lutze-Mann L, Lock RB. Dexamethasone resistance in B-cell precursor childhood acute lymphoblastic leukemia occurs downstream of ligand-induced nuclear translocation of the glucocorticoid receptor. Blood. 2005;105:2519–2526. - PubMed
-
- Bae J, Leo CP, Hsu SY, Hsueh AJ. MCL-1S, a splicing variant of the antiapoptotic BCL-2 family member MCL-1, encodes a proapoptotic protein possessing only the BH3 domain. J Biol Chem. 2000;275:25255–25261. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

