Chemosensory cues to conspecific emotional stress activate amygdala in humans
- PMID: 19641623
- PMCID: PMC2713432
- DOI: 10.1371/journal.pone.0006415
Chemosensory cues to conspecific emotional stress activate amygdala in humans
Abstract
Alarm substances are airborne chemical signals, released by an individual into the environment, which communicate emotional stress between conspecifics. Here we tested whether humans, like other mammals, are able to detect emotional stress in others by chemosensory cues. Sweat samples collected from individuals undergoing an acute emotional stressor, with exercise as a control, were pooled and presented to a separate group of participants (blind to condition) during four experiments. In an fMRI experiment and its replication, we showed that scanned participants showed amygdala activation in response to samples obtained from donors undergoing an emotional, but not physical, stressor. An odor-discrimination experiment suggested the effect was primarily due to emotional, and not odor, differences between the two stimuli. A fourth experiment investigated behavioral effects, demonstrating that stress samples sharpened emotion-perception of ambiguous facial stimuli. Together, our findings suggest human chemosensory signaling of emotional stress, with neurobiological and behavioral effects.
Conflict of interest statement
Figures
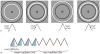
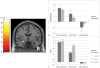
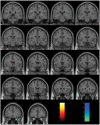

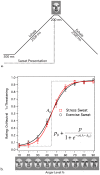
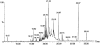

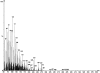
References
-
- Pfeiffer W. Alarm substances. Experientia. 1963;19:113–123. - PubMed
-
- Kiyokawa Y, Shimozuru M, Kikusui T, Takeuchi Y, Mori Y. Alarm pheromone increases defensive and risk assessment behaviors in male rats. Physiol Behav. 2006;87:383–387. - PubMed
-
- Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Mapping the neural circuit activated by alarm pheromone perception by c-Fos immunohistochemistry. Brain Res. 2005;1043:145–154. - PubMed
-
- Inagaki H, Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Enhancement of the acoustic startle reflex by an alarm pheromone in male rats. Physiol Behav. 2008;93:606–611. - PubMed
-
- Wysocki CJ, Preti G. Facts, fallacies, fears, and frustrations with human pheromones. Anat Rec A Discov Mol Cell Evol Biol. 2004;281:1201–1211. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

