TNF-alpha mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations
- PMID: 19641635
- PMCID: PMC2716944
TNF-alpha mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations
Abstract
Purpose: The pathophysiology of diabetic retinopathy involves leukocyte adhesion to retinal vasculature, early blood-retinal barrier breakdown, capillary nonperfusion, and endothelial cell death. We investigated the involvement of tumor necrosis factor alpha (TNF-alpha) in diabetes-related histopathological changes in two relevant rodent models.
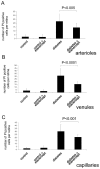
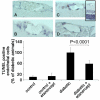
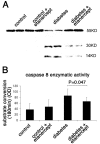
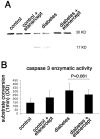
Methods: In short-term studies, Long-Evans rats with streptozotocin-induced diabetes were treated with or without the TNF-alpha inhibitor, etanercept. For long-term studies, tumor necrosis factor receptor I (TNF-RI)-deficient mice and TNF-RII-deficient mice, as well as C57/Bl6 wild-type mice, were fed 30% galactose for up to 20 months. The retinal histopathological alterations of hypergalactosemia were analyzed in trypsin digest preparations. Endothelial cell injury and apoptosis in rat retinas were evaluated by propidium iodide, TUNEL, CytoDeath staining, and DNA fragmentation ELISA. Caspase 3 and 8 activity was evaluated by immunoblotting and quantitative enzymatic activity assay.
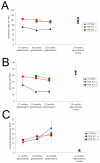
Results: Etanercept suppressed caspase activation, retinal cell injury, and apoptosis in short-term diabetic rats. Pericyte and endothelial cell loss were also reduced in long-term hypergalactosemic mice. Long-term studies demonstrated that pericyte loss and endothelial cell loss were reduced in comparison to wild-type diabetic controls.
Conclusions: Our study identifies an important role for TNF-alpha in the pathogenesis of signature diabetic retinopathy pathologies and demonstrates that etanercept can inhibit retinal cell death and long-term complication of diabetes. Taken together, our results suggest that etanercept could prove beneficial in preventing both early and late vascular diabetic complications.
Figures
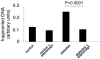


References
-
- Engerman RL, Kern TS. Retinopathy in animal models of diabetes. Diabetes Metab Rev. 1995;11:109–20. - PubMed
-
- Kern TS, Engerman RL. Galactose-induced retinal microangiopathy in rats. Invest Ophthalmol Vis Sci. 1995;36:490–6. - PubMed
-
- Kern TS, Engerman RL. A mouse model of diabetic retinopathy. Arch Ophthalmol. 1996;114:986–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
