Memantine lowers amyloid-beta peptide levels in neuronal cultures and in APP/PS1 transgenic mice
- PMID: 19642202
- PMCID: PMC2783840
- DOI: 10.1002/jnr.22172
Memantine lowers amyloid-beta peptide levels in neuronal cultures and in APP/PS1 transgenic mice
Abstract
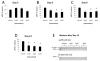
Memantine is a moderate-affinity, uncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist that stabilizes cognitive, functional, and behavioral decline in patients with moderate to severe Alzheimer's disease (AD). In AD, the extracellular deposition of fibrillogenic amyloid-beta peptides (Abeta) occurs as a result of aberrant processing of the full-length Abeta precursor protein (APP). Memantine protects neurons from the neurotoxic effects of Abeta and improves cognition in transgenic mice with high brain levels of Abeta. However, it is unknown how memantine protects cells against neurodegeneration and affects APP processing and Abeta production. We report the effects of memantine in three different systems. In human neuroblastoma cells, memantine, at therapeutically relevant concentrations (1-4 muM), decreased levels of secreted APP and Abeta(1-40). Levels of the potentially amylodogenic Abeta(1-42) were undetectable in these cells. In primary rat cortical neuronal cultures, memantine treatment lowered Abeta(1-42) secretion. At the concentrations used, memantine treatment was not toxic to neuroblastoma or primary cultures and increased cell viability and/or metabolic activity under certain conditions. In APP/presenilin-1 (PS1) transgenic mice exhibiting high brain levels of Abeta(1-42), oral dosing of memantine (20 mg/kg/day for 8 days) produced a plasma drug concentration of 0.96 microM and significantly reduced the cortical levels of soluble Abeta(1-42). The ratio of Abeta(1-40)/Abeta(1-42) increased in treated mice, suggesting effects on the gamma-secretase complex. Thus, memantine reduces the levels of Abeta peptides at therapeutic concentrations and may inhibit the accumulation of fibrillogenic Abeta in mammalian brains. Memantine's ability to preserve neuronal cells against neurodegeneration, to increase metabolic activity, and to lower Abeta level has therapeutic implications for neurodegenerative disorders.
Figures






References
-
- Bailey JA, Lahiri DK. Neuronal differentiation is accompanied by increased levels of SNAP-25 protein in fetal rat primary cortical neurons: implications in neuronal plasticity and Alzheimer’s disease. Ann N Y Acad Sci. 2006;1086:54–65. - PubMed
-
- Blanpied TA, Boeckman FA, Aizenman E, Johnson JW. Trapping channel block of NMDA-activated responses by amantadine and memantine. J Neurophysiol. 1997;77:309–323. - PubMed
-
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–76. - PubMed
-
- Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–276. - PubMed
-
- Chen HS, Lipton SA. The chemical biology of clinically tolerated NMDA receptor antagonists. J Neurochem. 2006;97:1611–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

