The P446L variant in GCKR associated with fasting plasma glucose and triglyceride levels exerts its effect through increased glucokinase activity in liver
- PMID: 19643913
- PMCID: PMC2758140
- DOI: 10.1093/hmg/ddp357
The P446L variant in GCKR associated with fasting plasma glucose and triglyceride levels exerts its effect through increased glucokinase activity in liver
Abstract
Genome-wide association studies have identified a number of signals for both Type 2 Diabetes and related quantitative traits. For the majority of loci, the transition from association signal to mutational mechanism has been difficult to establish. Glucokinase (GCK) regulates glucose storage and disposal in the liver where its activity is regulated by glucokinase regulatory protein (GKRP; gene name GCKR). Fructose-6 and fructose-1 phosphate (F6P and F1P) enhance or reduce GKRP-mediated inhibition, respectively. A common GCKR variant (P446L) is reproducibly associated with triglyceride and fasting plasma glucose levels in the general population. The aim of this study was to determine the mutational mechanism responsible for this genetic association. Recombinant human GCK and both human wild-type (WT) and P446L-GKRP proteins were generated. GCK kinetic activity was observed spectrophotometrically using an NADP(+)-coupled assay. WT and P446L-GKRP-mediated inhibition of GCK activity and subsequent regulation by phosphate esters were determined. Assays matched for GKRP activity demonstrated no difference in dose-dependent inhibition of GCK activity or F1P-mediated regulation. However, the response to physiologically relevant F6P levels was significantly attenuated with P446L-GKRP (n = 18; P <or= 0.03). Experiments using equimolar concentrations of both regulatory proteins confirmed these findings (n = 9; P < 0.001). In conclusion, P446L-GKRP has reduced regulation by physiological concentrations of F6P, resulting indirectly in increased GCK activity. Altered GCK regulation in liver is predicted to enhance glycolytic flux, promoting hepatic glucose metabolism and elevating concentrations of malonyl-CoA, a substrate for de novo lipogenesis, providing a mutational mechanism for the reported association of this variant with raised triglycerides and lower glucose levels.
Figures


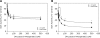

References
-
- Saxena R., Voight B.F., Lyssenko V., Burtt N.P., de Bakker P.I., Chen H., Roix J.J., Kathiresan S., Hirschhorn J.N., Daly M.J., et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–1336. - PubMed
-
- Orho-Melander M., Melander O., Guiducci C., Perez-Martinez P., Corella D., Roos C., Tewhey R., Rieder M.J., Hall J., Abecasis G., et al. A common missense variant in the glucokinase regulatory protein gene (GCKR) is associated with increased plasma triglyceride and C-reactive protein but lower fasting glucose concentrations. Diabetes. 2008;57:10. - PMC - PubMed
-
- Sparso T., Andersen G., Nielsen T., Burgdorf K.S., Gjesing A.P., Nielsen A.L., Albrechtsen A., Rasmussen S.S., Jorgensen T., Borch-Johnsen K., et al. The GCKR rs780094 polymorphism is associated with elevated fasting serum triacylglycerol, reduced fasting and OGTT-related insulinaemia, and reduced risk of type 2 diabetes. Diabetologia. 2008;51:70–75. - PubMed
-
- Vaxillaire M., Cavalcanti-Proenca C., Dechaume A., Tichet J., Marre M., Balkau B., Froguel P. The common P446L polymorphism in GCKR inversely modulates fasting glucose and triglyceride levels and reduces type 2 diabetes risk in the DESIR prospective general French population. Diabetes. 2008;57:2253–2257. - PMC - PubMed
-
- Matschinsky F.M. Glucokinase as glucose sensor and metabolic signal generator in pancreatic beta-cells and hepatocytes. Diabetes. 1990;39:647–652. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

