Luminal L-glutamate enhances duodenal mucosal defense mechanisms via multiple glutamate receptors in rats
- PMID: 19643955
- PMCID: PMC2763813
- DOI: 10.1152/ajpgi.90605.2008
Luminal L-glutamate enhances duodenal mucosal defense mechanisms via multiple glutamate receptors in rats
Abstract
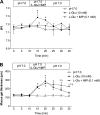
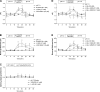
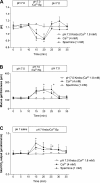
Presence of taste receptor families in the gastrointestinal mucosa suggests a physiological basis for local and early detection of a meal. We hypothesized that luminal L-glutamate, which is the primary nutrient conferring fundamental umami or proteinaceous taste, influences mucosal defense mechanisms in rat duodenum. We perfused the duodenal mucosa of anesthetized rats with L-glutamate (0.1-10 mM). Intracellular pH (pH(i)) of the epithelial cells, blood flow, and mucus gel thickness (MGT) were simultaneously and continuously measured in vivo. Some rats were pretreated with indomethacin or capsaicin. Duodenal bicarbonate secretion (DBS) was measured with flow-through pH and CO(2) electrodes. We tested the effects of agonists or antagonists for metabotropic glutamate receptor (mGluR) 1 or 4 or calcium-sensing receptor (CaSR) on defense factors. Luminal L-glutamate dose dependently increased pH(i) and MGT but had no effect on blood flow in the duodenum. L-glutamate (10 mM)-induced cellular alkalinization and mucus secretion were inhibited by pretreatment with indomethacin or capsaicin. L-glutamate effects on pH(i) and MGT were mimicked by mGluR4 agonists and inhibited by an mGluR4 antagonist. CaSR agonists acidified cells with increased MGT and DBS, unlike L-glutamate. Perfusion of L-glutamate with inosinate (inosine 5'-monophosphate, 0.1 mM) enhanced DBS only in combination, suggesting synergistic activation of the L-glutamate receptor, typical of taste receptor type 1. L-leucine or L-aspartate had similar effects on DBS without any effect on pH(i) and MGT. Preperfusion of L-glutamate prevented acid-induced cellular injury, suggesting that L-glutamate protects the mucosa by enhancing mucosal defenses. Luminal L-glutamate may activate multiple receptors and afferent nerves and locally enhance mucosal defenses to prevent subsequent injury attributable to acid exposure in the duodenum.
Figures







References
-
- Akiba Y, Furukawa O, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Acute adaptive cellular base uptake in rat duodenal epithelium. Am J Physiol Gastrointest Liver Physiol 280: G1083–G1092, 2001 - PubMed
-
- Akiba Y, Furukawa O, Guth PH, Engel E, Nastaskin I, Kaunitz JD. Sensory pathways and cyclooxygenase regulate mucus gel thickness in rat duodenum. Am J Physiol Gastrointest Liver Physiol 280: G470–G474, 2001 - PubMed
-
- Akiba Y, Ghayouri S, Takeuchi T, Mizumori M, Guth PH, Engel E, Swenson ER, Kaunitz JD. Carbonic anhydrases and mucosal vanilloid receptors help mediate the hyperemic response to luminal CO2 in rat duodenum. Gastroenterology 131: 142–152, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

