SIGNR1-negative red pulp macrophages protect against acute streptococcal sepsis after Leishmania donovani-induced loss of marginal zone macrophages
- PMID: 19644016
- PMCID: PMC2731129
- DOI: 10.2353/ajpath.2009.090258
SIGNR1-negative red pulp macrophages protect against acute streptococcal sepsis after Leishmania donovani-induced loss of marginal zone macrophages
Abstract
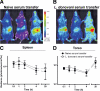
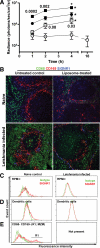
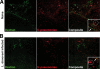
Marginal zone macrophages in the murine spleen play an important role in the capture of blood-borne pathogens and are viewed as an essential component of host defense against the development of pneumococcal sepsis. However, we and others have previously described the loss of marginal zone macrophages associated with the splenomegaly that follows a variety of viral and protozoal infections; this finding raises the question of whether these infected mice would become more susceptible to secondary pneumococcal infection. Contrary to expectations, we found that mice lacking marginal zone macrophages resulting from Leishmania donovani infection have increased resistance to Streptococcus pneumoniae type 3 and do not develop sepsis. Using biophotonic imaging, we observed that pneumococci are rapidly trapped in the spleens of L. donovani-infected mice. By selective depletion studies using clodronate liposomes, depleting monoclonal antibodies specific for Ly6C/G and Ly6G, and CD11c-DTR mice, we show that the enhanced early resistance in L. donovani-infected mice is entirely due to the activity of SIGNR1(-) red pulp macrophages. Our data demonstrate, therefore, that the normal requirement for SIGNR1(+) marginal zone macrophages to protect against a primary pneumococcal infection can, under conditions of splenomegaly, be readily compensated for by activated red pulp macrophages.
Figures


References
-
- Aichele P, Zinke J, Grode L, Schwendener RA, Kaufmann SH, Seiler P. Macrophages of the splenic marginal zone are essential for trapping of blood-borne particulate antigen but dispensable for induction of specific T cell responses. J Immunol. 2003;171:1148–1155. - PubMed
-
- Seiler P, Aichele P, Odermatt B, Hengartner H, Zinkernagel RM, Schwendener RA. Crucial role of marginal zone macrophages and marginal zone metallophils in the clearance of lymphocytic choriomeningitis virus infection. Eur J Immunol. 1997;27:2626–2633. - PubMed
-
- Gorak PM, Engwerda CR, Kaye PM. Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur J Immunol. 1998;28:687–695. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

