Rapid, point-of-care extraction of human immunodeficiency virus type 1 proviral DNA from whole blood for detection by real-time PCR
- PMID: 19644129
- PMCID: PMC2725643
- DOI: 10.1128/JCM.r00092-09
Rapid, point-of-care extraction of human immunodeficiency virus type 1 proviral DNA from whole blood for detection by real-time PCR
Abstract
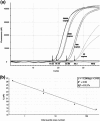
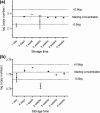
PCR detection of human immunodeficiency virus type 1 (HIV-1) proviral DNA is the method recommended for use for the diagnosis of HIV-1 infection in infants in limited-resource settings. Currently, testing must be performed in central laboratories, which are usually located some distance from health care facilities. While the collection and transportation of samples, such as dried blood spots, has improved test accessibility, the results are often not returned for several weeks. To enable PCR to be performed at the point of care while the mothers wait, we have developed a vertical filtration method that uses a separation membrane and an absorbent pad to extract cellular DNA from whole blood in less than 2 min. Cells are trapped in the separation membrane as the specimen is collected, and then a lysis buffer is added. The membrane retains the DNA, while the buffer washes away PCR inhibitors, which get wicked into the absorbent blotter pad. The membrane containing the entrapped DNA is then added to the PCR mixture without further purification. The method demonstrates a high degree of reproducibility and analytical sensitivity and allows the quantification of as few as 20 copies of HIV-1 proviral DNA from 100 microl of blood. In a blinded study with 182 longitudinal samples from infants (ages, 0 to 72 weeks) obtained from the Women and Infants Transmission Study, our assay demonstrated a sensitivity of 99% and a specificity of 100%.
Figures

References
-
- Beck, I. A., K. D. Drennan, A. J. Melvin, K. M. Mohan, A. M. Herz, J. Alarcon, J. Piscoya, C. Velazquez, and L. M. Frenkel. 2001. Simple, sensitive and specific detection of human immunodeficiency virus type 1 subtype B DNA in dried blood samples for diagnosis in infants in the field. J. Clin. Microbiol. 3929-33. - PMC - PubMed
-
- Bryson, Y. J., S. Pang, L. S. Wei, R. Dickover, A. Diagne, and I. S. Chen. 1995. Clearance of HIV infection in a perinatally infected infant. N. Engl. J. Med. 332833-838. - PubMed
-
- CDC. 2007. HIV AIDS surveillance report—cases of HIV infection and AIDS in the United States and dependent areas, vol. 19. Centers for Disease Control and Prevention, Atlanta, GA.
-
- Craig, M. H., R. W. Snow, and D. le Sueur. 1999. A climate-based distribution model of malaria transmission in sub-Saharan Africa. Parasitol. Today 15105-111. - PubMed
-
- Creek, T. L., G. G. Sherman, J. Nkengasong, L. Lu, T. Finkbeiner, M. G. Fowler, E. Rivadeneira, and N. Shaffer. 2007. Infant human immunodeficiency virus diagnosis in resource-limited settings: issues, technologies, and country experiences. Am. J. Obstet. Gynecol. 197S64-S71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

