Impact of HAART and CNS-penetrating antiretroviral regimens on HIV encephalopathy among perinatally infected children and adolescents
- PMID: 19644348
- PMCID: PMC2821205
- DOI: 10.1097/QAD.0b013e32832dc041
Impact of HAART and CNS-penetrating antiretroviral regimens on HIV encephalopathy among perinatally infected children and adolescents
Abstract
Objectives: Prior to antiretroviral treatment, HIV-infected children frequently developed encephalopathy, resulting in debilitating morbidity and mortality. This is the first large study to evaluate the impact of HAART and central nervous system (CNS)-penetrating antiretroviral regimens on the incidence of HIV encephalopathy and survival after diagnosis of HIV encephalopathy among perinatally infected children.
Design: A total of 2398 perinatally HIV-infected children with at least one neurological examination were followed in a US-based prospective cohort study conducted from 1993 to 2007.
Methods: Trends in incidence rates over calendar time were described and Cox regression models were used to estimate the effects of time-varying HAART and CNS-penetrating antiretroviral regimens on HIV encephalopathy and on survival after diagnosis of HIV encephalopathy.
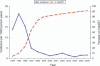
Results: During a median of 6.4 years of follow-up, 77 incident cases of HIV encephalopathy occurred [incidence rate 5.1 cases per 1000 person-years, 95% confidence interval (CI) 4.0-6.3]. A 10-fold decline in incidence was observed beginning in 1996, followed by a stable incidence rate after 2002. HAART regimens were associated with a 50% decrease (95% CI 14-71%) in the incidence of HIV encephalopathy compared with non-HAART regimens. High CNS-penetrating regimens were associated with a substantial survival benefit (74% reduction in the risk of death, 95% CI 39-89%) after HIV encephalopathy diagnosis compared with low CNS-penetrating regimens.
Conclusion: A dramatic decrease in the incidence of HIV encephalopathy occurred after the introduction of HAART. The use of HAART was highly effective in reducing the incidence of HIV encephalopathy among perinatally infected children and adolescents. Effective CNS-penetrating antiretroviral regimens are important in affecting survival after diagnosis of HIV encephalopathy.
2009 Wolters Kluwer Health | Lippincott Williams & Wilkins
Figures
Comment in
-
HIV, the brain, children, HAART and 'neuro-HAART': a complex mix.AIDS. 2009 Sep 10;23(14):1909-10. doi: 10.1097/QAD.0b013e32832ec4c6. AIDS. 2009. PMID: 19550290 No abstract available.
References
-
- Epstein LG, Sharer LR, Joshi VV, Fojas MM, Koenigsberger MR, Oleske JM. Progressive encephalopathy in children with acquired immune deficiency syndrome. Ann Neurol. 1985;17:488–496. - PubMed
-
- Belman AL, Ultmann MH, Horoupian D, Novick B, Spiro AJ, Rubinstein A, et al. Neurological complications in infants and children with acquired immune deficiency syndrome. Ann Neurol. 1985;18:560–566. - PubMed
-
- Epstein LG, Sharer LR, Oleske JM, Connor EM, Goudsmit J, Bagdon L, et al. Neurologic manifestations of human immunodeficiency virus infection in children. Pediatrics. 1986;78:678–687. - PubMed
-
- Epstein LG, Sharer LR, Goudsmit J. Neurological and neuropathological features of human immunodeficiency virus infection in children. Ann Neurol. 1988;23(Suppl):S19–S23. - PubMed
-
- Revision of the CDC surveillance case definition for acquired immunodeficiency syndrome. MMWR Morb Mortal Wkly Rep. 1987;36(1S Suppl):3S–15S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical