AVAPROMISE: A randomized clinical trial for increasing adherence through behavioural modification in essential hypertension
- PMID: 19644587
- PMCID: PMC2716987
AVAPROMISE: A randomized clinical trial for increasing adherence through behavioural modification in essential hypertension
Abstract
Background: Patients with hypertension often do not adhere to their medications.
Objective: To improve medication adherence in patients with essential hypertension by modifying their behaviours.
Patients and methods: From general practice settings, 4864 patients with essential hypertension were recruited and randomly assigned to receive the angiotensin receptor blocker irbesartan (Avapro) with (intervention group) or without (nonintervention group) a behavioural modification program (Avapromise) based on a model of change. Patients were followed up for 12 months. Patients were subgrouped based on their stage of change in the behavioural change continuum, and the intervention was tailored to address the needs of the particular subgroup. The primary efficacy measure was rate and time to discontinuation with irbesartan.
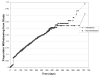
Results: At the end of the study, there was no significant difference in the discontinuation rates between the intervention (25.4%, 95% CI 23.7 to 27.2) and nonintervention (25.5%, 95% CI 23.8 to 27.3) groups (P=0.94). The time to discontinuation (P=0.87) and the extrapolated rate of discontinuation estimated from the Kaplan-Meir curve (intervention 23.1%, 95% CI 21.3 to 24.8; nonintervention 23.5%, 95% CI 21.8 to 25.3) were not different between the groups.
Conclusions: This behavioural modification intervention based on a model of change was not efficacious at increasing rates of adherence in patients with essential hypertension in this setting. More individualized interventions may be required to increase adherence in this population.
Keywords: Angiotensin; Behaviour modification; Hypertension.
Figures
References
-
- Hennekens C. Lessons from hypertension trials. Am J Med. 1998;104:50S–3S. - PubMed
-
- Dawber T. The Framingham study: The epidemiology of atherosclerotic disease. Cambridge: Harvard University Press; 1980.
-
- Collins R, Peto R, MacMohan S, et al. Blood pressure, stroke and coronary heart disease. Part 2. Short-term reductions in blood pressure: overview of randomized drug trials in their epidemiological context. Lancet. 1990;335:827–38. - PubMed
-
- Joffres M, Ghadirian P, Fodor G, et al. Awareness, treatment, and control of hypertension in Canada. Am J Hypertens. 1997;10:1097–102. - PubMed
-
- Waeber B, Burnier M, Brunner H. How to improve adherence with prescribed treatment in hypertensive patients? J Cardiovasc Pharmacol. 2000;35(Suppl 3):S23–6. - PubMed
LinkOut - more resources
Full Text Sources