The partial substrate dethiaacetyl-coenzyme A mimics all critical carbon acid reactions in the condensation half-reaction catalyzed by Thermoplasma acidophilum citrate synthase
- PMID: 19645419
- PMCID: PMC2765034
- DOI: 10.1021/bi9006447
The partial substrate dethiaacetyl-coenzyme A mimics all critical carbon acid reactions in the condensation half-reaction catalyzed by Thermoplasma acidophilum citrate synthase
Abstract
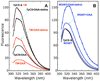
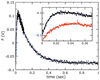
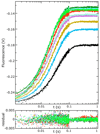
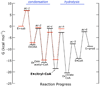
Citrate synthase (CS) performs two half-reactions: the mechanistically intriguing condensation of acetyl-CoA with oxaloacetate (OAA) to form citryl-CoA and the subsequent, slower hydrolysis of citryl-CoA that generally dominates steady-state kinetics. The condensation reaction requires the abstraction of a proton from the methyl carbon of acetyl-CoA to generate a reactive enolate intermediate. The carbanion of that intermediate then attacks the OAA carbonyl to furnish citryl-CoA, the initial product. Using stopped-flow and steady-state fluorescence methods, kinetic substrate isotope effects, and mutagenesis of active site residues, we show that all of the processes that occur in the condensation half-reaction performed by Thermoplasma acidophilum citrate synthase (TpCS) with the natural thioester substrate, acetyl-CoA, also occur with the ketone inhibitor dethiaacetyl-CoA. Free energy profiles demonstrate that the nonhydrolyzable product of the condensation reaction, dethiacitryl-CoA, forms a particularly stable complex with TpCS but not pig heart CS.
Figures







Similar articles
-
Kinetics and mechanism of the citrate synthase from the thermophilic archaeon Thermoplasma acidophilum.Biochemistry. 2000 Mar 7;39(9):2283-96. doi: 10.1021/bi991982r. Biochemistry. 2000. PMID: 10694395
-
Photophysics of tryptophan fluorescence: link with the catalytic strategy of the citrate synthase from Thermoplasma acidophilum.Biochemistry. 2005 Feb 8;44(5):1394-413. doi: 10.1021/bi048323l. Biochemistry. 2005. PMID: 15683225
-
Effects of changes in three catalytic residues on the relative stabilities of some of the intermediates and transition states in the citrate synthase reaction.Biochemistry. 1998 Jul 7;37(27):9724-37. doi: 10.1021/bi980325g. Biochemistry. 1998. PMID: 9657685
-
Ability of single-site mutants of citrate synthase to catalyze proton transfer from the methyl group of dethiaacetyl-coenzyme A, a non-thioester substrate analog.Biochemistry. 1997 Apr 1;36(13):3981-90. doi: 10.1021/bi963058s. Biochemistry. 1997. PMID: 9092828
-
The Novel Role of Mitochondrial Citrate Synthase and Citrate in the Pathophysiology of Alzheimer's Disease.J Alzheimers Dis. 2023;94(s1):S453-S472. doi: 10.3233/JAD-220514. J Alzheimers Dis. 2023. PMID: 37393492 Free PMC article. Review.
Cited by
-
Mutational analysis of Saccharomyces cerevisiae Erf2 reveals a two-step reaction mechanism for protein palmitoylation by DHHC enzymes.J Biol Chem. 2010 Dec 3;285(49):38104-14. doi: 10.1074/jbc.M110.169102. Epub 2010 Sep 17. J Biol Chem. 2010. PMID: 20851885 Free PMC article.
-
Common enzymological experiments allow free energy profile determination.Biochemistry. 2013 Aug 27;52(34):5952-65. doi: 10.1021/bi400696j. Epub 2013 Aug 16. Biochemistry. 2013. PMID: 23906433 Free PMC article.
-
An active site-tail interaction in the structure of hexahistidine-tagged Thermoplasma acidophilum citrate synthase.Acta Crystallogr F Struct Biol Commun. 2015 Oct;71(Pt 10):1292-9. doi: 10.1107/S2053230X15015939. Epub 2015 Sep 23. Acta Crystallogr F Struct Biol Commun. 2015. PMID: 26457521 Free PMC article.
References
-
- Guynn RW, Gelberg HJ, Veech RL. Equilibrium constants of the malate dehydrogenase, citrate synthase, citrate lyase, and acetyl coenzyme A hydrolysis reactions under physiological conditions. J. Biol. Chem. 1973;248:6957–6965. - PubMed
-
- Pettersson G, Lill U, Eggerer H. Mechanism of interaction of citrate synthase with citryl-CoA. Eur. J. Biochem. 1989;182:119–124. - PubMed
-
- Kurz LC, Drysdale G, Riley M, Tomar MA, Chen J, Russell RJM, Danson MJ. Kinetics and mechanism of the citrate synthase from the thermophilic archaeon Thermoplasma acidophilum. Biochemistry. 2000;39:2283–2296. - PubMed
-
- Russell RJM, Hough DW, Danson MJ, Taylor GL. The crystal structure of citrate synthase from the thermophilic archaeon, Thermoplasma acidophilum. Structure. 1994;2:1157–1167. - PubMed
-
- Evans CT, Kurz LC, Remington SJ, Srere PA. Active site mutants of pig citrate synthase: effects of mutations on the enzyme catalytic and structural properties. Biochemistry. 1996;35:10661–10672. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

