Palladium-catalyzed regio-, diastereo-, and enantioselective benzylic allylation of 2-substituted pyridines
- PMID: 19645450
- PMCID: PMC3235048
- DOI: 10.1021/ja904441a
Palladium-catalyzed regio-, diastereo-, and enantioselective benzylic allylation of 2-substituted pyridines
Abstract
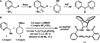
We report a new method for the highly regio-, diastereo-, and enantioselective palladium-catalyzed allylic alkylation of 2-substituted pyridines that allows for the formation of homoallylic stereocenters containing alkyl, aryl, heteroaryl, and nitrogen substituents. When the reaction is conducted with asymmetric acyclic electrophiles, both linear and branched products may be obtained exclusively by selecting the appropriate regioisomeric starting material and ligand, an example of the "memory effect." Deuterium-labeling studies reveal that though no such phenomenon occurs with racemic cyclic electrophiles, the chiral ligand employed reacts kinetically faster with the enantiomer of the substrate for which it is "matched" and yet eventually converts all "mismatched" substrate to product.
Figures
References
-
-
Ketones: Braun B, Laicher F, Meier T. Angew. Chem. 2000;112:3637. Trost BM, Schroeder GM. Chem. Eur. J. 2004;11:174. Trost BM, Xu J, Reichle M. J. Am. Chem. Soc. 2007;129:282. Zheng WH, Zheng BH, Zhang Y, Hou XL. J. Am. Chem. Soc. 2007;129:7718. Trost BM, Xu J, Schmidt T. J. Am. Chem. Soc. 2008;130:11852. β-keto esters: Trost BM, Sacchi KL, Schroeder GM, Asakawa N. Org. Lett. 2002;4:3427. Imino esters: Baldwin IC, Williams JMJ. Tetrahedron: Asymmetry. 1995;6:1515. Nakoji M, Kanayama T, Okino T, Takemoto Y. J. Org. Chem. 2002;67:7418. Azalactones: Trost BM, Ariza X. Angew. Chem. Int. Ed. Engl. 1997;36:2635. Trost BM, Lee CB. J. Am. Chem. Soc. 1998;120:6818. Nitroalkanes: Trost BM, Surivet J-P. J. Am. Chem. Soc. 2000;122:6291.
-
-
- Trost BM, Thaisrivongs DA. J. Am. Chem. Soc. 2008;130:14092. - PubMed
-
-
Interestingly, the 1-naphthyl analog of 3g could be obtained in 100% conversion by 1H NMR but only 2:1 dr.
-
-
-
No reaction was observed with the unmethylated carbamate or the corresponding phthalimide-protected aminopyridine.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources