Kinetic and structural investigations of the allosteric site in human epithelial 15-lipoxygenase-2
- PMID: 19645454
- PMCID: PMC2746553
- DOI: 10.1021/bi9009242
Kinetic and structural investigations of the allosteric site in human epithelial 15-lipoxygenase-2
Abstract
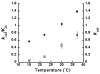
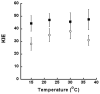
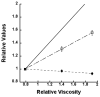
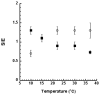
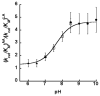
Allosteric regulation of human lipoxygenase (hLO) activity has recently been implicated in the cellular biology of prostate cancer. In the current work, we present isotope effect, pH, and substrate inhibitor data of epithelial 15-hLO-2, which probe the allosteric effects on its mechanistic behavior. The Dk(cat)/KM for 15-hLO-2, with AA and LA as substrate, is large indicating hydrogen atom abstraction is the principle rate-determining step, involving a tunneling mechanism for both substrates. For AA, there are multiple rate determining steps (RDS) at both high and low temperatures, with both diffusion and hydrogen bonding rearrangements contributing at high temperature, but only hydrogen bonding rearrangements contributing at low temperature. The observed kinetic dependency on the hydrogen bonding rearrangement is eliminated upon addition of the allosteric effector, 13-(S)-hydroxyoctadecadienoic acid (13-HODE), and no allosteric effects were seen on diffusion or hydrogen atom abstraction. The (k(cat)/KM)AA/(k(cat)/KM)LA ratio was observed to have a pH dependence, which was fit with a titration curve (pKa = 7.7), suggesting the protonation of a histidine residue, which could hydrogen bond with the carboxylate of 13-HODE. Assuming this interaction, 13-HODE was docked to the solvent exposed histidines of a 15-hLO-2 homology model and found to bind well with H627, suggesting a potential location for the allosteric site. Utilizing d31-LA as an inhibitor, it was demonstrated that the binding of d31-LA to the allosteric site changes the conformation of 15-hLO-2 such that the affinity for substrate increases. This result suggests that allosteric binding locks the enzyme into a catalytically competent state, which facilitates binding of LA and decreases the (k(cat)/KM)AA/(k(cat)/KM)LA ratio. Finally, the magnitude of the 13-HODE KD for 15-hLO-2 is over 200-fold lower than that of 13-HODE for 15-hLO-1, changing the substrate specificity of 15-hLO-2 to 1.9. This would alter the LO product distribution and increase the production of the pro-tumorigenic, 13-HODE, possibly representing a pro-tumorigenic feedback loop for 13-HODE and 15-hLO-2.
Figures

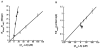
Similar articles
-
Substrate specificity changes for human reticulocyte and epithelial 15-lipoxygenases reveal allosteric product regulation.Biochemistry. 2008 Jul 15;47(28):7364-75. doi: 10.1021/bi800550n. Epub 2008 Jun 21. Biochemistry. 2008. PMID: 18570379 Free PMC article.
-
Mechanistic investigations of human reticulocyte 15- and platelet 12-lipoxygenases with arachidonic acid.Biochemistry. 2009 Jul 7;48(26):6259-67. doi: 10.1021/bi802332j. Biochemistry. 2009. PMID: 19469483 Free PMC article.
-
Kinetic investigations of the rate-limiting step in human 12- and 15-lipoxygenase.Biochemistry. 2003 May 13;42(18):5236-43. doi: 10.1021/bi0273462. Biochemistry. 2003. PMID: 12731864
-
Kinetic and structural investigations into the allosteric and pH effect on the substrate specificity of human epithelial 15-lipoxygenase-2.Biochemistry. 2013 Nov 12;52(45):8026-35. doi: 10.1021/bi4010649. Epub 2013 Oct 30. Biochemistry. 2013. PMID: 24171444 Free PMC article.
-
Structural and Functional Biology of Mammalian ALOX Isoforms with Particular Emphasis on Enzyme Dimerization and Their Allosteric Properties.Int J Mol Sci. 2024 Nov 9;25(22):12058. doi: 10.3390/ijms252212058. Int J Mol Sci. 2024. PMID: 39596127 Free PMC article. Review.
Cited by
-
Arachidonate 15-lipoxygenase type B: Regulation, function, and its role in pathophysiology.Front Pharmacol. 2022 Nov 9;13:1042420. doi: 10.3389/fphar.2022.1042420. eCollection 2022. Front Pharmacol. 2022. PMID: 36438817 Free PMC article. Review.
-
Comparative kinetic isotope effects on first- and second-order rate constants of soybean lipoxygenase variants uncover a substrate-binding network.J Biol Chem. 2019 Nov 29;294(48):18069-18076. doi: 10.1074/jbc.RA119.010826. Epub 2019 Oct 17. J Biol Chem. 2019. PMID: 31624150 Free PMC article.
-
Biosynthesis of the Maresin Intermediate, 13S,14S-Epoxy-DHA, by Human 15-Lipoxygenase and 12-Lipoxygenase and Its Regulation through Negative Allosteric Modulators.Biochemistry. 2020 May 19;59(19):1832-1844. doi: 10.1021/acs.biochem.0c00233. Epub 2020 May 7. Biochemistry. 2020. PMID: 32324389 Free PMC article.
-
Fatty acids negatively regulate platelet function through formation of noncanonical 15-lipoxygenase-derived eicosanoids.Pharmacol Res Perspect. 2023 Feb;11(1):e01056. doi: 10.1002/prp2.1056. Pharmacol Res Perspect. 2023. PMID: 36708179 Free PMC article.
-
Hydrogen tunneling in a prokaryotic lipoxygenase.Biochemistry. 2014 Apr 15;53(14):2212-4. doi: 10.1021/bi500070q. Epub 2014 Apr 3. Biochemistry. 2014. PMID: 24641705 Free PMC article.
References
-
- Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. - PubMed
-
- Capdevila JH, Falck JR, Estabrook RW. Cytochrome P450 and the arachidonate cascade. FASEB J. 1992;6:731–736. - PubMed
-
- Yamamoto S. Mammalian lipoxygenases: molecular structures and functions. Biochim Biophys Acta. 1992;1128:117–131. - PubMed
-
- Solomon EI, Zhou J, Neese F, Pavel EG. New insights from spectroscopy into the structure/function relationships of lipoxygenases. Chem Biol. 1997;4:795–808. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

