Allylic C-H amination for the preparation of syn-1,3-amino alcohol motifs
- PMID: 19645489
- PMCID: PMC2751616
- DOI: 10.1021/ja9054959
Allylic C-H amination for the preparation of syn-1,3-amino alcohol motifs
Abstract
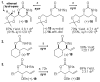
A highly selective and general Pd/sulfoxide-catalyzed allylic C-H amination reaction en route to syn-1,3-amino alcohol motifs is reported. Key to achieving this reactivity under mild conditions is the use of electron-deficient N-nosyl carbamate nucleophiles that are thought to promote functionalization by furnishing higher concentrations of anionic species in situ. The reaction is shown to be orthogonal to classical C-C bond-forming/-reduction sequences as well as nitrene-based C-H amination methods.
Figures


References
-
- List B. J Am Chem Soc. 2000;122:9336.
- Wenzel AG, Jacobsen EN. J Am Chem Soc. 2002;124:12964. - PubMed
- Kochi T, Tang TP, Ellman JA. J Am Chem Soc. 2003;125:11276. - PubMed
- Matsunaga S, Yoshida T, Morimoto H, Kumagai N, Shibasaki M. J Am Chem Soc. 2004;126:8777. - PubMed
- Josephsohn NS, Snapper ML, Hoveyda AH. J Am Chem Soc. 2004;126:3734. - PubMed
- Enders D, Grondal C, Vrettou M, Raabe G. Angew Chem Int Ed. 2005;44:4079. - PubMed
-
-
For some recent examples of asymmetric conjugate addition reactions with nitrogen nucleophiles to furnish β-amino ketones see: Myers JK, Jacobsen EN. J Am Chem Soc. 1999;121:8959.Guerin DJ, Miller SJ. J Am Chem Soc. 2002;124:2134.Sibi MP, Prabagaran N, Ghorpade SG, Jasperse CP. J Am Chem Soc. 2003;125:11796.Yamigawa N, Qin H, Matsunaga S, Shibasaki M. J Am Chem Soc. 2005;127:13419.Chen YK, Yoshida M, MacMillan DWC. J Am Chem Soc. 2006;128:9328.
-
-
-
Espino CG, Wehn PM, Chow J, Du Bois J. J Am Chem Soc. 2001;123:6935.Fiori KW, Fleming JJ, Du Bois J. Angew Chem Int Ed. 2004;43:4349.A sulfamate product analogous to 4 was generated via a chemoselective Rh-nitrene based reaction in 43% yield: Zalatan DN, Du Bois J. J Am Chem Soc. 2008;130:9220.Milczek E, Boudet N, Blakey S. Angew Chem Int Ed. 2008;47:6825.Liang JL, Yuan SX, Huang JS, Yu WY, Che CM. Angew Chem Int Ed. 2002;41:3465.
-
-
-
Interestingly, high chemoselectivities and reactivities have been reported for intermolecular Rh-nitrene allylic aminations of internal olefins: Liang C, Collet F, Robert-Peillard F, Müller P, Dodd RH, Dauban P. J Am Chem Soc. 2008;130:343.
-
-
-
Linear allylic C—H acetoxylation: Chen MS, White MC. J Am Chem Soc. 2004;126:1346.Branched allylic C—H esterification: Chen MS, Prabagaran N, Labenz NA, White MC. J Am Chem Soc. 2005;127:6970.Oxidative C—H macrolactonization: Fraunhoffer KJ, Prabagaran N, Sirois LE, White MC. J Am Chem Soc. 2006;128:9032.For a linear allylic acetoxylation reaction using DMA solvent see: Mistudome T, Umetani T, Nosaka N, Mori K, Mizugaki T, Ebitani K, Kaneda K. Angew Chem Int Ed. 2006;45:481.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

