How vestibular neurons solve the tilt/translation ambiguity. Comparison of brainstem, cerebellum, and thalamus
- PMID: 19645876
- PMCID: PMC2860452
- DOI: 10.1111/j.1749-6632.2009.03939.x
How vestibular neurons solve the tilt/translation ambiguity. Comparison of brainstem, cerebellum, and thalamus
Abstract
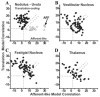
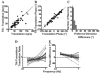
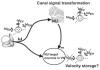
The peripheral vestibular system is faced by a sensory ambiguity, where primary otolith afferents respond identically to translational (inertial) accelerations and changes in head orientation relative to gravity. Under certain conditions, this sensory ambiguity can be resolved using extra-otolith cues, including semicircular canal signals. Here we review and summarize how neurons in the vestibular nuclei, rostral fastigial nuclei, cerebellar nodulus/uvula, and thalamus respond during combinations of tilt and translation. We focus primarily on cerebellar cortex responses, as nodulus/uvula Purkinje cells reliably encode translation rather than net gravito-inertial acceleration. In contrast, neurons in the vestibular and rostral fastigial nuclei, as well as the ventral lateral and ventral posterior nuclei of the thalamus represent a continuum, with some encoding translation and some net gravito-inertial acceleration. This review also outlines how Purkinje cells use semicircular canal signals to solve the ambiguity problem and how this solution fails at low frequencies. We conclude by attempting to bridge the gap between the proposed roles of nodulus/uvula in tilt/translation discrimination and velocity storage.
Conflict of interest statement
Figures

References
-
- Angelaki DE, et al. Neurons compute internal models of the physical laws of motion. Nature. 2004;430:560–564. - PubMed
-
- Dickman JD, Angelaki DE, Correia MJ. Response properties of gerbil otolith afferents to small angle pitch and roll tilts. Brain Res. 1991;556:303–310. - PubMed
-
- Fernandez C, Goldberg JM, Abend WK. Response to static tilts of peripheral neurons innervating otolith organs of the squirrel monkey. J Neurophysiol. 1972;35:978–987. - PubMed
-
- Merfeld DM, Zupan L, Peterka RJ. Humans use internal models to estimate gravity and linear acceleration. Nature. 1999;398:615–618. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

