A class of DNA-binding peptides from wheat bud causes growth inhibition, G2 cell cycle arrest and apoptosis induction in HeLa cells
- PMID: 19646247
- PMCID: PMC2726120
- DOI: 10.1186/1476-4598-8-55
A class of DNA-binding peptides from wheat bud causes growth inhibition, G2 cell cycle arrest and apoptosis induction in HeLa cells
Abstract
Background: Deproteinized DNA from eukaryotic and prokaryotic cells still contains a low-molecular weight peptidic fraction which can be dissociated by alkalinization of the medium. This fraction inhibits RNA transcription and tumor cell growth. Removal from DNA of normal cells causes amplification of DNA template activity. This effect is lower or absent in several cancer cell lines. Likewise, the amount of active peptides in cancer cell DNA extracts is lower than in DNA preparation of the corresponding normal cells. Such evidence, and their ubiquitous presence, suggests that they are a regulatory, conserved factor involved in the control of normal cell growth and gene expression.
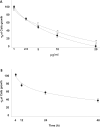
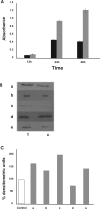
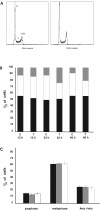
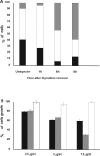
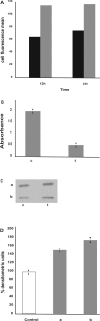
Results: We report that peptides extracted from wheat bud chromatin induce growth inhibition, G2 arrest and caspase-dependent apoptosis in HeLa cells. The growth rate is decreased in cells treated during the S phase only and it is accompanied by DNA damage and DNA synthesis inhibition. In G2 cells, this treatment induces inactivation of the CDK1-cyclin B1 complex and an increase of active chk1 kinase expression.
Conclusion: The data indicate that the chromatin peptidic pool inhibits HeLa cell growth by causing defective DNA replication which, in turn, arrests cell cycle progression to mitosis via G2 checkpoint pathway activation.
Figures
Similar articles
-
A pool of peptides extracted from wheat bud chromatin inhibits tumor cell growth by causing defective DNA synthesis.Cell Div. 2013 Aug 6;8:11. doi: 10.1186/1747-1028-8-11. eCollection 2013. Cell Div. 2013. PMID: 23915323 Free PMC article.
-
Cdk5 activator-binding protein C53 regulates apoptosis induced by genotoxic stress via modulating the G2/M DNA damage checkpoint.J Biol Chem. 2005 May 27;280(21):20651-9. doi: 10.1074/jbc.M413431200. Epub 2005 Mar 24. J Biol Chem. 2005. PMID: 15790566
-
Genistein-induced neuronal apoptosis and G2/M cell cycle arrest is associated with MDC1 up-regulation and PLK1 down-regulation.Eur J Pharmacol. 2007 Dec 1;575(1-3):12-20. doi: 10.1016/j.ejphar.2007.07.039. Epub 2007 Jul 28. Eur J Pharmacol. 2007. PMID: 17706963
-
GADD45-induced cell cycle G2-M arrest associates with altered subcellular distribution of cyclin B1 and is independent of p38 kinase activity.Oncogene. 2002 Dec 12;21(57):8696-704. doi: 10.1038/sj.onc.1206034. Oncogene. 2002. PMID: 12483522
-
Regulation of the G2/M transition by p53.Oncogene. 2001 Apr 5;20(15):1803-15. doi: 10.1038/sj.onc.1204252. Oncogene. 2001. PMID: 11313928 Review.
Cited by
-
A pool of peptides extracted from wheat bud chromatin inhibits tumor cell growth by causing defective DNA synthesis.Cell Div. 2013 Aug 6;8:11. doi: 10.1186/1747-1028-8-11. eCollection 2013. Cell Div. 2013. PMID: 23915323 Free PMC article.
-
Plant-Derived Natural Compounds in Genetic Vaccination and Therapy for HPV-Associated Cancers.Cancers (Basel). 2020 Oct 23;12(11):3101. doi: 10.3390/cancers12113101. Cancers (Basel). 2020. PMID: 33114220 Free PMC article. Review.
-
βTrCP facilitates MRN complex localization on chromatin to enhance DNA repair.Commun Biol. 2025 Jul 11;8(1):1044. doi: 10.1038/s42003-025-08462-5. Commun Biol. 2025. PMID: 40646298 Free PMC article.
-
Natural Bioactives: Back to the Future in the Fight against Human Papillomavirus? A Narrative Review.J Clin Med. 2022 Mar 7;11(5):1465. doi: 10.3390/jcm11051465. J Clin Med. 2022. PMID: 35268556 Free PMC article. Review.
References
-
- Werner D, Neuer-Nitsche B. Discontinuities of peptide nature in DNA. In: Bekhor I, Mirell CJ, Liew CC, editor. Progress in nonhistone protein research. Vol. 3. Boca Raton FL: CRC Press; 1989. pp. 99–118.
-
- Coderoni S, Miano A, Bramucci M, Felici F, Paparelli M, Amici D, Gianfranceschi GL. Isolation and characterization of small phosphorylated peptides controlling transcription "in vitro" from trout testis chromatin DNA. Physiol Chem Phys Med. 1988;20:91–108. - PubMed
-
- Manera E, Manchiotti L, Lugaro G. An improved method for the isolation of deprimerones from spermatozoa chromatin. IRCS Med Sci. 1984;12:745–746.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

