Vasodilator factors in the systemic and local adaptations to pregnancy
- PMID: 19646248
- PMCID: PMC2739214
- DOI: 10.1186/1477-7827-7-79
Vasodilator factors in the systemic and local adaptations to pregnancy
Abstract
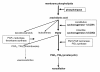
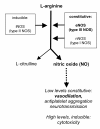
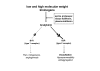
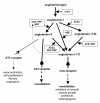
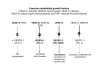
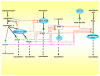
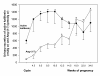
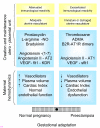
We postulate that an orchestrated network composed of various vasodilatory systems participates in the systemic and local hemodynamic adaptations in pregnancy. The temporal patterns of increase in the circulating and urinary levels of five vasodilator factors/systems, prostacyclin, nitric oxide, kallikrein, angiotensin-(1-7) and VEGF, in normal pregnant women and animals, as well as the changes observed in preeclamptic pregnancies support their functional role in maintaining normotension by opposing the vasoconstrictor systems. In addition, the expression of these vasodilators in the different trophoblastic subtypes in various species supports their role in the transformation of the uterine arteries. Moreover, their expression in the fetal endothelium and in the syncytiotrophoblast in humans, rats and guinea-pigs, favour their participation in maintaining the uteroplacental circulation. The findings that sustain the functional associations of the various vasodilators, and their participation by endocrine, paracrine and autocrine regulation of the systemic and local vasoactive changes of pregnancy are abundant and compelling. However, further elucidation of the role of the various players is hampered by methodological problems. Among these difficulties is the complexity of the interactions between the different factors, the likelihood that experimental alterations induced in one system may be compensated by the other players of the network, and the possibility that data obtained by manipulating single factors in vitro or in animal studies may be difficult to translate to the human. In addition, the impossibility of sampling the uteroplacental interface along normal pregnancy precludes obtaining longitudinal profiles of the various players. Nevertheless, the possibility of improving maternal blood pressure regulation, trophoblast invasion and uteroplacental flow by enhancing vasodilation (e.g. L-arginine, NO donors, VEGF transfection) deserves unravelling the intricate association of vasoactive factors and the systemic and local adaptations to pregnancy.
Figures

References
-
- Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69:1–7. - PubMed
-
- Kaufmann P, Mayhew TM, Charnock-Jones DS. Aspects of human fetoplacental vasculogenesis and angiogenesis. II. Changes during normal pregnancy. Placenta. 2004;25:114–126. - PubMed
-
- Reynolds LP, Redmer DA. Angiogenesis in the placenta. Biol Reprod. 2001;64:1033–1040. - PubMed
-
- Hytten F. Blood volume changes in normal pregnancy. Clin Haematol. 1985;14:601–612. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

