Differential activities of thalidomide and isoprenoid biosynthetic pathway inhibitors in multiple myeloma cells
- PMID: 19646757
- PMCID: PMC4228479
- DOI: 10.1016/j.leukres.2009.06.035
Differential activities of thalidomide and isoprenoid biosynthetic pathway inhibitors in multiple myeloma cells
Abstract
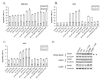
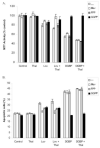
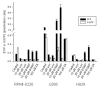
Thalidomide has emerged as an effective agent for treating multiple myeloma, however the precise mechanism of action remains unknown. Agents known to target the isoprenoid biosynthetic pathway (IBP) can have cytotoxic effects in myeloma cells. The interactions between thalidomide and IBP inhibitors in human multiple myeloma cells were evaluated. Enhanced cytotoxicity and induction of apoptosis were observed in RPMI-8226 cells. Examination of intracellular levels of farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP) revealed a wide variance in basal levels and response to IBP inhibitors. These findings provide a mechanism for the differential sensitivity of myeloma cells to pharmacologic manipulation of the IBP.
Copyright (c) 2009 Elsevier Ltd. All rights reserved.
Figures



Similar articles
-
In vivo evaluation of combination therapy targeting the isoprenoid biosynthetic pathway.Pharmacol Res. 2021 May;167:105528. doi: 10.1016/j.phrs.2021.105528. Epub 2021 Mar 3. Pharmacol Res. 2021. PMID: 33667685 Free PMC article.
-
Targeting the Isoprenoid Biosynthetic Pathway in Multiple Myeloma.Int J Mol Sci. 2022 Dec 21;24(1):111. doi: 10.3390/ijms24010111. Int J Mol Sci. 2022. PMID: 36613550 Free PMC article. Review.
-
Mechanisms for autophagy modulation by isoprenoid biosynthetic pathway inhibitors in multiple myeloma cells.Oncotarget. 2015 Dec 8;6(39):41535-49. doi: 10.18632/oncotarget.6365. Oncotarget. 2015. PMID: 26595805 Free PMC article.
-
Effects of farnesyl pyrophosphate accumulation on calvarial osteoblast differentiation.Endocrinology. 2011 Aug;152(8):3113-22. doi: 10.1210/en.2011-0016. Epub 2011 May 17. Endocrinology. 2011. PMID: 21586555
-
Inhibition of farnesyl pyrophosphate (FPP) and/or geranylgeranyl pyrophosphate (GGPP) biosynthesis and its implication in the treatment of cancers.Crit Rev Biochem Mol Biol. 2019 Feb;54(1):41-60. doi: 10.1080/10409238.2019.1568964. Epub 2019 Feb 18. Crit Rev Biochem Mol Biol. 2019. PMID: 30773935 Review.
Cited by
-
Structural Insight into Geranylgeranyl Diphosphate Synthase (GGDPS) for Cancer Therapy.Mol Cancer Ther. 2024 Jan 3;23(1):14-23. doi: 10.1158/1535-7163.MCT-23-0358. Mol Cancer Ther. 2024. PMID: 37756579 Free PMC article. Review.
-
In vivo evaluation of combination therapy targeting the isoprenoid biosynthetic pathway.Pharmacol Res. 2021 May;167:105528. doi: 10.1016/j.phrs.2021.105528. Epub 2021 Mar 3. Pharmacol Res. 2021. PMID: 33667685 Free PMC article.
-
Targeting the Isoprenoid Biosynthetic Pathway in Multiple Myeloma.Int J Mol Sci. 2022 Dec 21;24(1):111. doi: 10.3390/ijms24010111. Int J Mol Sci. 2022. PMID: 36613550 Free PMC article. Review.
-
Isoprenoid biosynthetic pathway inhibition disrupts monoclonal protein secretion and induces the unfolded protein response pathway in multiple myeloma cells.Leuk Res. 2011 Apr;35(4):551-9. doi: 10.1016/j.leukres.2010.08.008. Epub 2010 Sep 9. Leuk Res. 2011. PMID: 20828814 Free PMC article.
-
Chemical genetic control of cytokine signaling in CAR-T cells using lenalidomide-controlled membrane-bound degradable IL-7.Leukemia. 2024 Mar;38(3):590-600. doi: 10.1038/s41375-023-02113-6. Epub 2023 Dec 20. Leukemia. 2024. PMID: 38123696 Free PMC article.
References
-
- Hideshima T, Chauhan D, Shima Y, Raje N, Davies FE, Tai YT, Treon SP, Lin B, Schlossman RL, Richardson P. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96:2943–2950. others. - PubMed
-
- Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Richardson PG, Hideshima T, Munshi NC, Treon SP, Anderson KC. Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood. 2002;99:4525–4530. - PubMed
-
- Davies FE, Raje N, Hideshima T, Lentzsch S, Young G, Tai YT, Lin B, Podar K, Gupta D, Chauhan D. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98:210–216. others. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

