A blood-borne PDGF/VEGF-like ligand initiates wound-induced epidermal cell migration in Drosophila larvae
- PMID: 19646875
- PMCID: PMC2783944
- DOI: 10.1016/j.cub.2009.07.019
A blood-borne PDGF/VEGF-like ligand initiates wound-induced epidermal cell migration in Drosophila larvae
Abstract
Epidermal cell migration is critical for restoration of tissue structure and function after damage. However, the mechanisms by which differentiated cells neighboring the wound sense the wound and assume a motile phenotype remain unclear. Here, we show that Pvr, a receptor tyrosine kinase (RTK) related to platelet-derived growth factor (PDGF) and vascular endothelial growth factor (VEGF) receptors, and one of its ligands, Pvf1, are required for epidermal wound closure. Morphological comparison of wound-edge cells lacking Pvr or the Jun N-terminal kinase (JNK) signaling pathway previously implicated in larval wound closure suggests that Pvr signaling leads wound-margin epidermal cells to extend actin-based cell processes into the wound gap while JNK mediates transient dedifferentiation of cells at the wound margin. Genetic epistasis experiments reinforce the conclusion that the JNK and Pvr signaling pathways act in parallel. Tissue-specific knockdown and rescue experiments suggest that epidermally derived Pvf1 may be sequestered in the blood and that tissue damage exposes blood-borne Pvf1 to Pvr receptors on wound-edge epidermal cells and initiates the extension of cell processes into the wound gap. These results uncover a novel mechanism of sensing tissue damage and suggest that PDGF/VEGF ligands and receptors may play a conserved autocrine role in epidermal wound closure.
Figures
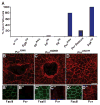

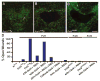
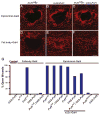

Similar articles
-
Two ligands signal through the Drosophila PDGF/VEGF receptor to ensure proper salivary gland positioning.Mech Dev. 2007 Jul;124(6):441-8. doi: 10.1016/j.mod.2007.03.003. Epub 2007 Mar 13. Mech Dev. 2007. PMID: 17462868 Free PMC article.
-
PVF1/PVR signaling and apoptosis promotes the rotation and dorsal closure of the Drosophila male terminalia.Int J Dev Biol. 2004 Dec;48(10):1087-94. doi: 10.1387/ijdb.041859am. Int J Dev Biol. 2004. PMID: 15602694
-
Pvr and distinct downstream signaling factors are required for hemocyte spreading and epidermal wound closure at Drosophila larval wound sites.G3 (Bethesda). 2022 Jan 4;12(1):jkab388. doi: 10.1093/g3journal/jkab388. G3 (Bethesda). 2022. PMID: 34751396 Free PMC article.
-
Signaling pathways directing the movement and fusion of epithelial sheets: lessons from dorsal closure in Drosophila.Differentiation. 2002 Jun;70(4-5):181-203. doi: 10.1046/j.1432-0436.2002.700408.x. Differentiation. 2002. PMID: 12147138 Review.
-
Functions and mechanisms of receptor tyrosine kinase Torso signaling: lessons from Drosophila embryonic terminal development.Dev Dyn. 2005 Mar;232(3):656-72. doi: 10.1002/dvdy.20295. Dev Dyn. 2005. PMID: 15704136 Free PMC article. Review.
Cited by
-
A Pvr-AP-1-Mmp1 signaling pathway is activated in astrocytes upon traumatic brain injury.Elife. 2024 Oct 31;12:RP87258. doi: 10.7554/eLife.87258. Elife. 2024. PMID: 39480704 Free PMC article.
-
Simvastatin preparations promote PDGF-BB secretion to repair LPS-induced endothelial injury through the PDGFRβ/PI3K/Akt/IQGAP1 signalling pathway.J Cell Mol Med. 2019 Dec;23(12):8314-8327. doi: 10.1111/jcmm.14709. Epub 2019 Oct 1. J Cell Mol Med. 2019. PMID: 31576676 Free PMC article.
-
Long-term In Vivo Tracking of Inflammatory Cell Dynamics Within Drosophila Pupae.J Vis Exp. 2018 Jun 14;(136):57871. doi: 10.3791/57871. J Vis Exp. 2018. PMID: 29985351 Free PMC article.
-
Immune response in the barrier epithelia: lessons from the fruit fly Drosophila melanogaster.J Innate Immun. 2012;4(3):273-83. doi: 10.1159/000332947. Epub 2012 Jan 10. J Innate Immun. 2012. PMID: 22237424 Free PMC article. Review.
-
The role of transcription-independent damage signals in the initiation of epithelial wound healing.Nat Rev Mol Cell Biol. 2013 Apr;14(4):249-62. doi: 10.1038/nrm3541. Epub 2013 Feb 27. Nat Rev Mol Cell Biol. 2013. PMID: 23443750 Review.
References
-
- Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276:75–81. - PubMed
-
- Li G, Gustafson-Brown C, Hanks SK, Nason K, Arbeit JM, Pogliano K, Wisdom RM, Johnson RS. c-Jun Is Essential for Organization of the Epidermal Leading Edge. Developmental Cell. 2003;4:865–877. - PubMed
-
- Shirakata Y, Kimura R, Nanba D, Iwamoto R, Tokumaru S, Morimoto C, Yokota K, Nakamura M, Sayama K, Mekada E, et al. Heparin-binding EGF-like growth factor accelerates keratinocyte migration and skin wound healing. J Cell Sci. 2005;118:2363–2370. - PubMed
-
- Werner S, Smola H, Liao X, Longaker MT, Krieg T, Hofschneider PH, Williams LT. The function of KGF in morphogenesis of epithelium and reepithelialization of wounds. Science. 1994;266:819–822. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

