Characterizing the initial encounter complex in cadherin adhesion
- PMID: 19646884
- PMCID: PMC4113602
- DOI: 10.1016/j.str.2009.06.012
Characterizing the initial encounter complex in cadherin adhesion
Abstract
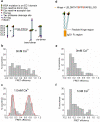
Cadherins are Ca(2+)-dependent cell-cell adhesion proteins with an extracellular region of five domains (EC1 to EC5). Adhesion is mediated by "strand swapping" of a conserved tryptophan residue in position 2 between EC1 domains of opposing cadherins, but the formation of this structure is not well understood. Using single-molecule fluorescence resonance energy transfer and single-molecule force measurements with the atomic force microscope, we demonstrate that cadherins initially interact via EC1 domains without swapping tryptophan-2 to form a weak Ca(2+) dependent initial encounter complex that has 25% of the bond strength of a strand-swapped dimer. We suggest that cadherin dimerization proceeds via an induced fit mechanism where the monomers first form a tryptophan-2 independent initial encounter complex and then undergo subsequent conformational changes to form the final strand-swapped dimer.
Figures


Comment in
-
How do adhesion proteins stick?Structure. 2009 Aug 12;17(8):1035-6. doi: 10.1016/j.str.2009.07.003. Structure. 2009. PMID: 19679080
References
-
- Al-Amoudi A, Diez DC, Betts MJ, Frangakis AS. The molecular architecture of cadherins in native epidermal desmosomes. Nature. 2007;450:832–U838. - PubMed
-
- Boggon TJ, Murray J, Chappuis-Flament S, Wong E, Gumbiner BM, Shapiro L. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science. 2002;296:1308–1313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

