Phosphorylation of Mcm2 by Cdc7 promotes pre-replication complex assembly during cell-cycle re-entry
- PMID: 19647517
- PMCID: PMC2725784
- DOI: 10.1016/j.molcel.2009.06.014
Phosphorylation of Mcm2 by Cdc7 promotes pre-replication complex assembly during cell-cycle re-entry
Abstract
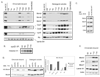
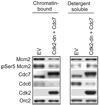
Cyclin E has been shown to have a role in pre-replication complex (Pre-RC) assembly in cells re-entering the cell cycle from quiescence. The assembly of the pre-RC, which involves the loading of six MCM subunits (Mcm2-7), is a prerequisite for DNA replication. We found that cyclin E, through activation of Cdk2, promotes Mcm2 loading onto chromatin. This function is mediated in part by promoting the accumulation of Cdc7 messenger RNA and protein, which then phosphorylates Mcm2. Consistent with this, a phosphomimetic mutant of Mcm2 can bypass the requirement for Cdc7 in terms of Mcm2 loading. Furthermore, ectopic expression of both Cdc6 and Cdc7 can rescue the MCM loading defect associated with expression of dominant-negative Cdk2. These results are consistent with a role for cyclin E-Cdk2 in promoting the accumulation of Cdc6 and Cdc7, which is required for Mcm2 loading when cells re-enter the cell cycle from quiescence.
Figures





References
-
- Bochman ML, Schwacha A. The Mcm2–7 complex has in vitro elicase activity. Mol Cell. 2008;31:287–293. - PubMed
-
- Boulet AM, Moon AM, Arenkiel BR, Capecchi MR. The roles of Fgf4 and Fgf8 in limb bud initiation and outgrowth. Dev Biol. 2004;273:361–372. - PubMed
-
- Cook JG, Chasse DA, Nevins JR. The regulated association of Cdt1 with minichromosome maintenance proteins and Cdc6 in mammalian cells. J Biol Chem. 2004;279:9625–9633. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

