mosR, a novel transcriptional regulator of hypoxia and virulence in Mycobacterium tuberculosis
- PMID: 19648248
- PMCID: PMC2747884
- DOI: 10.1128/JB.00778-09
mosR, a novel transcriptional regulator of hypoxia and virulence in Mycobacterium tuberculosis
Abstract
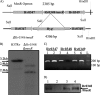
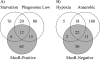
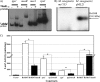
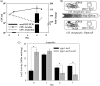
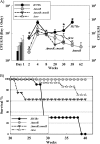
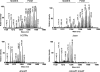
Latent tuberculosis represents a high-risk burden for one-third of the world population. Previous analysis of murine tuberculosis identified a novel transcriptional regulator encoded by Rv0348 that could control the establishment of persistent tuberculosis. Disruption of the Rv0348 gene from the genome of the virulent H37Rv strain of Mycobacterium tuberculosis revealed a global impact on the transcriptional profiles of 163 genes, including induction of the mammalian cell entry (mce1) operon and the repression of a significant number of genes involved in hypoxia and starvation responses. Nonetheless, gel shift assays did not reveal direct binding between Rv0348 and a set of regulated promoters, suggesting an indirect regulatory role. However, when expressed in Mycobacterium smegmatis, the Rv0348 transcripts were significantly responsive to different levels of hypoxia and the encoded protein was shown to regulate genes involved in hypoxia [e.g., Rv3130c (tgs1)] and intracellular survival (e.g., mce1), among other genes. Interestingly, the colonization level of the DeltamosR mutant strain was significantly lower than that of the wild-type strain of M. tuberculosis, suggesting its attenuation in the murine model of tuberculosis. Taken together, our analyses indicated that the Rv0348 gene encodes a novel transcriptional factor that regulates several operons involved in mycobacterial survival, especially during hypoxia; hence, we propose that Rv0348 be renamed mosR for regulator of mycobacterial operons of survival.
Figures


References
-
- Andreu, N., and I. Gibert. 2008. Cell population heterogeneity in Mycobacterium tuberculosis H37Rv. Tuberculosis 88:553-559. - PubMed
-
- Bardarov, S., M. S. Pavelka, V. Sambandamurthy, M. Larsen, J. Tufariello, J. Chan, G. Hatfull, and W. R. Jacobs. 2002. Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology 148:3007-3017. - PubMed
-
- Betts, J. C., P. T. Lukey, L. C. Robb, R. A. McAdam, and K. Duncan. 2002. Evaluation of a nutrient starvation model of Mycobacterium tuberculosis persistence by gene and protein expression profiling. Mol. Microbiol. 43:717-731. - PubMed
-
- Braunstein, M., S. S. Bardarov, and W. R. Jacobs. 2002. Genetic methods for deciphering virulence determinants of Mycobacterium tuberculosis. Methods Enzymol. 358:67-99. - PubMed
-
- Camacho, L. R., P. Constant, C. Raynaud, M. A. Laneelle, J. A. Triccas, B. Gicquel, M. Daffe, and C. Guilhot. 2001. Analysis of the phthiocerol dimycocerosate locus of Mycobacterium tuberculosis: evidence that this lipid is involved in the cell wall permeability barrier. J. Biol. Chem. 276:19845-19854. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

