Chemotropic guidance facilitates axonal regeneration and synapse formation after spinal cord injury
- PMID: 19648914
- PMCID: PMC2753201
- DOI: 10.1038/nn.2365
Chemotropic guidance facilitates axonal regeneration and synapse formation after spinal cord injury
Abstract
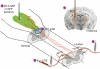
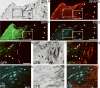
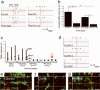
A principal objective of spinal cord injury (SCI) research is the restoration of axonal connectivity to denervated targets. We tested the hypothesis that chemotropic mechanisms would guide regenerating spinal cord axons to appropriate brainstem targets. We subjected rats to cervical level 1 (C1) lesions and combinatorial treatments to elicit axonal bridging into and beyond lesion sites. Lentiviral vectors expressing neurotrophin-3 (NT-3) were then injected into an appropriate brainstem target, the nucleus gracilis, and an inappropriate target, the reticular formation. NT-3 expression in the correct target led to reinnervation of the nucleus gracilis in a dose-related fashion, whereas NT-3 expression in the reticular formation led to mistargeting of regenerating axons. Axons regenerating into the nucleus gracilis formed axodendritic synapses containing rounded vesicles, reflective of pre-injury synaptic architecture. Thus, we report for the first time, to the best of our knowledge, the reinnervation of brainstem targets after SCI and an essential role for chemotropic axon guidance in target selection.
Figures




Comment in
-
Reconnecting injured nerves.Nat Neurosci. 2009 Sep;12(9):1085. doi: 10.1038/nn0909-1085. Nat Neurosci. 2009. PMID: 19710648 No abstract available.
References
-
- McQuarrie IG, Grafstein B, Gershon MD. Axonal regeneration in the rat sciatic nerve: effect of a conditioning lesion and of dbcAMP. Brain Res. 1977;132:443–453. - PubMed
-
- Schreyer DJ, Skene JH. Injury-associated induction of GAP-43 expression displays axon branch specificity in rat dorsal root ganglion neurons. J Neurobiol. 1993;24:959–970. - PubMed
-
- Chen DF, Schneider GE, Martinou JC, Tonegawa S. Bcl-2 promotes regeneration of severed axons in mammalian CNS. Nature. 1997;385:434–439. - PubMed
-
- Goldberg JL, Klassen MP, Hua Y, Barres BA. Amacrine-signaled loss of intrinsic axon growth ability by retinal ganglion cells. Science. 2002;296:1860–1864. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

