Evaluation of RTS,S/AS02A and RTS,S/AS01B in adults in a high malaria transmission area
- PMID: 19649245
- PMCID: PMC2714466
- DOI: 10.1371/journal.pone.0006465
Evaluation of RTS,S/AS02A and RTS,S/AS01B in adults in a high malaria transmission area
Abstract
Background: This study advances the clinical development of the RTS,S/AS01B candidate malaria vaccine to malaria endemic populations. As a primary objective it compares the safety and reactogenicity of RTS,S/AS01B to the more extensively evaluated RTS,S/AS02A vaccine.
Methodology: A Phase IIb, single centre, double-blind, controlled trial of 6 months duration with a subsequent 6 month single-blind follow-up conducted in Kisumu West District, Kenya between August 2005 and August 2006. 255 healthy adults aged 18 to 35 years were randomized (1ratio1ratio1) to receive 3 doses of RTS,S/AS02A, RTS,S/AS01B or rabies vaccine (Rabipur; Chiron Behring GmbH) at months 0, 1, 2. The primary objective was the occurrence of severe (grade 3) solicited or unsolicited general (i.e. systemic) adverse events (AEs) during 7 days follow up after each vaccination.
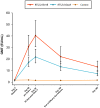
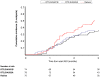
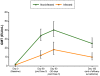
Principal findings: Both candidate vaccines had a good safety profile and were well tolerated. One grade 3 systemic AE occurred within 7 days of vaccination (RTS,S/AS01B group). No unsolicited AEs or SAEs were related to vaccine. A marked increase in anti-CS antibody GMTs was observed post Dose 2 of both RTS,S/AS01B (31.6 EU/mL [95% CI: 23.9 to 41.6]) and RTS,S/AS02A (16.7 EU/mL [95% CI: 12.9 to 21.7]). A further increase was observed post Dose 3 in both the RTS,S/AS01B (41.4 EU/mL [95% CI: 31.7 to 54.2]) and RTS,S/AS02A (21.4 EU/mL [95% CI: 16.0 to 28.7]) groups. Anti-CS antibody GMTs were significantly greater with RTS,S/AS01B compared to RTS,S/AS02A at all time points post Dose 2 and Dose 3. Both candidate vaccines produced strong anti-HBs responses. Vaccine efficacy in the RTS,S/AS01B group was 29.5% (95% CI: -15.4 to 56.9, p = 0.164) and in the RTS,S/AS02A group 31.7% (95% CI: -11.6 to 58.2, p = 0.128).
Conclusions: Both candidate malaria vaccines were well tolerated over a 12 month surveillance period. A more favorable immunogenicity profile was observed with RTS,S/AS01B than with RTS,S/AS02A.
Trial registration: Clinicaltrials.gov NCT00197054.
Conflict of interest statement
Figures

References
-
- Greenwood BM, Bojang K, Whitty CJM, Targett GAT. Malaria. Lancet. 2005;365:1487–98. - PubMed
-
- Breman JG. The ears of the hippopotamus: manifestations, determinants, and estimates of the malaria burden. Am J Trop Med Hyg. 2001;64:1–11. - PubMed
-
- Malaney P, Spielman A, Sachs J. The Malaria Gap. Am J Trop Med Hyg. 2004;71(supp2):141–146. - PubMed
-
- Tediosi F, Hutton G, Maire N, Smith TA, Ross A, et al. Predicting the cost-effectiveness of introducing a pre-erythrocytic malaria vaccine into the expanded program on immunization in Tanzania. Am J Trop Med Hyg. 2006;75(supp2):131–143. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

