Punicic acid a conjugated linolenic acid inhibits TNFalpha-induced neutrophil hyperactivation and protects from experimental colon inflammation in rats
- PMID: 19649246
- PMCID: PMC2714468
- DOI: 10.1371/journal.pone.0006458
Punicic acid a conjugated linolenic acid inhibits TNFalpha-induced neutrophil hyperactivation and protects from experimental colon inflammation in rats
Abstract
Background: Neutrophils play a major role in inflammation by releasing large amounts of ROS produced by NADPH-oxidase and myeloperoxidase (MPO). The proinflammatory cytokine TNFalpha primes ROS production through phosphorylation of the NADPH-oxidase subunit p47phox on Ser345. Conventional anti-inflammatory therapies remain partially successful and may have side effects. Therefore, regulation of neutrophil activation by natural dietary components represents an alternative therapeutic strategy in inflammatory diseases such as inflammatory bowel diseases. The aim of this study was to assess the effect of punicic acid, a conjugated linolenic fatty acid from pomegranate seed oil on TNFalpha-induced neutrophil hyperactivation in vitro and on colon inflammation in vivo.
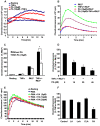
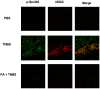
Methodology and principal findings: We analyzed the effect of punicic acid on TNFalpha-induced neutrophil upregulation of ROS production in vitro and on TNBS-induced rat colon inflammation. Results show that punicic acid inhibited TNFalpha-induced priming of ROS production in vitro while preserving formyl-methionyl-leucyl-phenylalanine (fMLP)-induced response. This effect was mediated by the inhibition of Ser345-p47phox phosphorylation and upstream kinase p38MAPK. Punicic acid also inhibited fMLP- and TNFalpha+fMLP-induced MPO extracellular release from neutrophils. In vivo experiments showed that punicic acid and pomegranate seed oil intake decreased neutrophil-activation and ROS/MPO-mediated tissue damage as measured by F2-isoprostane release and protected rats from TNBS-induced colon inflammation.
Conclusions/significance: These data show that punicic acid exerts a potent anti-inflammatory effect through inhibition of TNFalpha-induced priming of NADPH oxidase by targeting the p38MAPKinase/Ser345-p47phox-axis and MPO release. This natural dietary compound may provide a novel alternative therapeutic strategy in inflammatory diseases such as inflammatory bowel diseases.
Conflict of interest statement
Figures

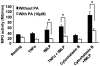




Similar articles
-
The prolyl isomerase Pin1 acts as a novel molecular switch for TNF-alpha-induced priming of the NADPH oxidase in human neutrophils.Blood. 2010 Dec 23;116(26):5795-802. doi: 10.1182/blood-2010-03-273094. Epub 2010 Oct 18. Blood. 2010. PMID: 20956805 Free PMC article.
-
Zymosan induces NADPH oxidase activation in human neutrophils by inducing the phosphorylation of p47phox and the activation of Rac2: involvement of protein tyrosine kinases, PI3Kinase, PKC, ERK1/2 and p38MAPkinase.Biochem Pharmacol. 2013 Jan 1;85(1):92-100. doi: 10.1016/j.bcp.2012.10.010. Epub 2012 Oct 22. Biochem Pharmacol. 2013. PMID: 23085266
-
Distinct ligand-dependent roles for p38 MAPK in priming and activation of the neutrophil NADPH oxidase.J Biol Chem. 2004 Jun 25;279(26):27059-68. doi: 10.1074/jbc.M314258200. Epub 2004 Apr 20. J Biol Chem. 2004. PMID: 15102856
-
Priming of the neutrophil NADPH oxidase activation: role of p47phox phosphorylation and NOX2 mobilization to the plasma membrane.Semin Immunopathol. 2008 Jul;30(3):279-89. doi: 10.1007/s00281-008-0118-3. Epub 2008 Jun 7. Semin Immunopathol. 2008. PMID: 18536919 Review.
-
Punicic acid: A striking health substance to combat metabolic syndromes in humans.Lipids Health Dis. 2017 May 30;16(1):99. doi: 10.1186/s12944-017-0489-3. Lipids Health Dis. 2017. PMID: 28558700 Free PMC article. Review.
Cited by
-
Plant-Derived Nutraceuticals and Immune System Modulation: An Evidence-Based Overview.Vaccines (Basel). 2020 Aug 22;8(3):468. doi: 10.3390/vaccines8030468. Vaccines (Basel). 2020. PMID: 32842641 Free PMC article. Review.
-
Grape, Pomegranate, Olive, and Tomato By-Products Fed to Dairy Ruminants Improve Milk Fatty Acid Profile without Depressing Milk Production.Foods. 2023 Feb 17;12(4):865. doi: 10.3390/foods12040865. Foods. 2023. PMID: 36832939 Free PMC article. Review.
-
Effect of pomegranate juice consumption on biochemical parameters and complete blood count.Exp Ther Med. 2017 Aug;14(2):1756-1762. doi: 10.3892/etm.2017.4690. Epub 2017 Jun 27. Exp Ther Med. 2017. PMID: 28781633 Free PMC article.
-
Assessment of the impact of microwave roasting on nutrient content, lipid profile, and oxidative stability of pomegranate seed oil.Food Chem X. 2024 Oct 5;24:101875. doi: 10.1016/j.fochx.2024.101875. eCollection 2024 Dec 30. Food Chem X. 2024. PMID: 39974711 Free PMC article.
-
Potential therapeutic effect of pomegranate seed oil on ovarian ischemia/reperfusion injury in rats.Iran J Basic Med Sci. 2018 Dec;21(12):1262-1268. doi: 10.22038/ijbms.2018.30149.7268. Iran J Basic Med Sci. 2018. PMID: 30627370 Free PMC article.
References
-
- Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. - PubMed
-
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. - PubMed
-
- Nathan CF. Respiratory burst in adherent human neutrophils: triggering by colony-stimulating factors CSF-GM and CSF-G. Blood. 1989;73:301–306. - PubMed
-
- Babior BM. Phagocytes and oxidative stress. Am J Med. 2000;109:33–44. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

