The viscoelastic properties of passive eye muscle in primates. II: testing the quasi-linear theory
- PMID: 19649257
- PMCID: PMC2715107
- DOI: 10.1371/journal.pone.0006480
The viscoelastic properties of passive eye muscle in primates. II: testing the quasi-linear theory
Abstract
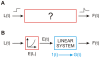
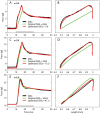
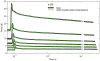
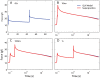
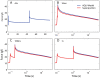
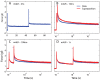
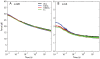
We have extensively investigated the mechanical properties of passive eye muscles, in vivo, in anesthetized and paralyzed monkeys. The complexity inherent in rheological measurements makes it desirable to present the results in terms of a mathematical model. Because Fung's quasi-linear viscoelastic (QLV) model has been particularly successful in capturing the viscoelastic properties of passive biological tissues, here we analyze this dataset within the framework of Fung's theory.We found that the basic properties assumed under the QLV theory (separability and superposition) are not typical of passive eye muscles. We show that some recent extensions of Fung's model can deal successfully with the lack of separability, but fail to reproduce the deviation from superposition.While appealing for their elegance, the QLV model and its descendants are not able to capture the complex mechanical properties of passive eye muscles. In particular, our measurements suggest that in a passive extraocular muscle the force does not depend on the entire length history, but to a great extent is only a function of the last elongation to which it has been subjected. It is currently unknown whether other passive biological tissues behave similarly.
Conflict of interest statement
Figures






Similar articles
-
The nonlinearity of passive extraocular muscles.Ann N Y Acad Sci. 2011 Sep;1233(1):17-25. doi: 10.1111/j.1749-6632.2011.06111.x. Ann N Y Acad Sci. 2011. PMID: 21950971 Free PMC article.
-
Creep behavior of passive bovine extraocular muscle.J Biomed Biotechnol. 2011;2011:526705. doi: 10.1155/2011/526705. Epub 2011 Nov 2. J Biomed Biotechnol. 2011. PMID: 22131809 Free PMC article.
-
The viscoelastic properties of passive eye muscle in primates. III: force elicited by natural elongations.PLoS One. 2010 Mar 8;5(3):e9595. doi: 10.1371/journal.pone.0009595. PLoS One. 2010. PMID: 20221406 Free PMC article.
-
The viscoelastic properties of passive eye muscle in primates. I: static forces and step responses.PLoS One. 2009;4(4):e4850. doi: 10.1371/journal.pone.0004850. Epub 2009 Apr 1. PLoS One. 2009. PMID: 19337381 Free PMC article.
-
Extraocular muscles: basic and clinical aspects of structure and function.Surv Ophthalmol. 1995 May-Jun;39(6):451-84. doi: 10.1016/s0039-6257(05)80055-4. Surv Ophthalmol. 1995. PMID: 7660301 Review.
Cited by
-
The nonlinearity of passive extraocular muscles.Ann N Y Acad Sci. 2011 Sep;1233(1):17-25. doi: 10.1111/j.1749-6632.2011.06111.x. Ann N Y Acad Sci. 2011. PMID: 21950971 Free PMC article.
-
Atomic force microscopy determination of Young׳s modulus of bovine extra-ocular tendon fiber bundles.J Biomech. 2014 Jun 3;47(8):1899-903. doi: 10.1016/j.jbiomech.2014.02.011. Epub 2014 Feb 14. J Biomech. 2014. PMID: 24767704 Free PMC article.
-
Characterization of ocular tissues using microindentation and hertzian viscoelastic models.Invest Ophthalmol Vis Sci. 2011 Jun 1;52(6):3475-82. doi: 10.1167/iovs.10-6867. Invest Ophthalmol Vis Sci. 2011. PMID: 21310907 Free PMC article.
-
A nonlinear model of passive muscle viscosity.J Biomech Eng. 2011 Sep;133(9):091007. doi: 10.1115/1.4004993. J Biomech Eng. 2011. PMID: 22010742 Free PMC article.
-
Creep behavior of passive bovine extraocular muscle.J Biomed Biotechnol. 2011;2011:526705. doi: 10.1155/2011/526705. Epub 2011 Nov 2. J Biomed Biotechnol. 2011. PMID: 22131809 Free PMC article.
References
-
- Buchthal F, Kaiser E. The rheology of the cross striated muscle fiber, with particular reference to isotonic conditions. Dan Biol Medd. 1951;21:1–318.
-
- Bagni MA, Cecchi G, Colombini B, Colomo F. Mechanical properties of frog muscle fibres at rest and during twitch contraction. J Electromyogr Kinesiol. 1999;9:77–86. - PubMed
-
- Mutungi G, Ranatunga KW. The visco-elasticity of resting intact mammalian (rat) fast muscle fibres. J Muscle Res Cell Motil. 1996;17:357–364. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

