Safety and efficacy of dihydroartemisinin-piperaquine in falciparum malaria: a prospective multi-centre individual patient data analysis
- PMID: 19649267
- PMCID: PMC2716525
- DOI: 10.1371/journal.pone.0006358
Safety and efficacy of dihydroartemisinin-piperaquine in falciparum malaria: a prospective multi-centre individual patient data analysis
Abstract
Background: The fixed dose antimalarial combination of dihydroartemisinin-piperaquine (DP) is a promising new artemisinin-based combination therapy (ACT). We present an individual patient data analysis of efficacy and tolerability in acute uncomplicated falciparum malaria, from seven published randomized clinical trials conducted in Africa and South East Asia using a predefined in-vivo protocol. Comparator drugs were mefloquine-artesunate (MAS3) in Thailand, Myanmar, Laos and Cambodia; artemether-lumefantrine in Uganda; and amodiaquine+sulfadoxine-pyrimethamine and artesunate+amodiaquine in Rwanda.
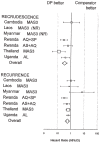
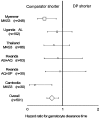
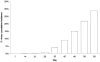
Methods and findings: In total 3,547 patients were enrolled: 1,814 patients (32% children under five years) received DP and 1,733 received a comparator antimalarial at 12 different sites and were followed for 28-63 days. There was no significant heterogeneity between trials. DP was well tolerated with 1.7% early vomiting. There were less adverse events with DP in children and adults compared to MAS3 except for diarrhea; ORs (95%CI) 2.74 (2.13 to 3.51) and 3.11 (2.31 to 4.18), respectively. DP treatment resulted in a rapid clearance of fever and parasitaemia. The PCR genotype corrected efficacy at Day 28 of DP assessed by survival analysis was 98.7% (95%CI 97.6-99.8). DP was superior to the comparator drugs in protecting against both P.falciparum recurrence and recrudescence (P = 0.001, weighted by site). There was no difference between DP and MAS3 in treating P. vivax co-infections and in suppressing the first relapse (median interval to P. vivax recurrence: 6 weeks). Children under 5 y were at higher risk of recurrence for both infections. The proportion of patients developing gametocytaemia (P = 0.002, weighted by site) and the subsequent gametocyte carriage rates were higher with DP (11/1000 person gametocyte week, PGW) than MAS3 (6/1000 PGW, P = 0.001, weighted by site).
Conclusions: DP proved a safe, well tolerated, and highly effective treatment of P.falciparum malaria in Asia and Africa, but the effect on gametocyte carriage was inferior to that of MAS3.
Conflict of interest statement
Figures

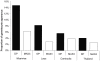

References
-
- WHO. 2006. Guidelines for the treatment of malaria (WHO/HTM/MAL/2006.1108), available at: http://www.who.int/malaria/docs/TreatmentGuidelines2006.pdf (page 17) [accessed 2 August 2007]
-
- http://www.who.int/malaria/treatmentpolicies.html [accessed 19 August 2008]
-
- Hien TT, Mai PP, Dolecek C, Phuong P, Dung NT, Truong NT, Thanh N, Thai LH, An DTH, Quyen NTH, White NJ, Farrar JJ. Dihydroartemisinin-piperaquine against multidrug resistant falciparum malaria in Viet Nam: randomized clinical trial. Lancet. 2004;363:18–22. - PubMed
-
- Tarning J, Ashley EA, Lindegardh N, Stepniewska K, Phaiphun L, et al. Population pharmacokinetics of piperaquine after two different treatment regimens of dihydroartemisinin-piperaquine in patients with acute uncomplicated Plasmodium falciparum malaria in Thailand. Antimicrob Agents Chemother. 2008;52:1052–61. - PMC - PubMed

