Development of budesonide microparticles using spray-drying technology for pulmonary administration: design, characterization, in vitro evaluation, and in vivo efficacy study
- PMID: 19649711
- PMCID: PMC2802174
- DOI: 10.1208/s12249-009-9290-6
Development of budesonide microparticles using spray-drying technology for pulmonary administration: design, characterization, in vitro evaluation, and in vivo efficacy study
Abstract
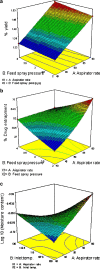
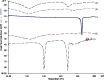
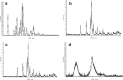
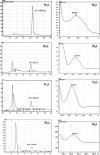
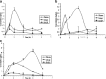
The purpose of this research was to generate, characterize, and investigate the in vivo efficacy of budesonide (BUD) microparticles prepared by spray-drying technology with a potential application as carriers for pulmonary administration with sustained-release profile and improved respirable fraction. Microspheres and porous particles of chitosan (drug/chitosan, 1:2) were prepared by spray drying using optimized process parameters and were characterized for different physicochemical parameters. Mass median aerodynamic diameter and geometric standard deviation for conventional, microspheres, and porous particles formulations were 2.75, 4.60, and 4.30 microm and 2.56, 1.75, and 2.54, respectively. Pharmacokinetic study was performed in rats by intratracheal administration of either placebo or developed dry powder inhalation (DPI) formulation. Pharmacokinetic parameters were calculated (Ka, Ke, T(max), C(max), AUC, and Vd) and these results indicated that developed formulations extended half life compared to conventional formulation with onefold to fourfold improved local and systemic bioavailability. Estimates of relative bioavailability suggested that developed formulations have excellent lung deposition characteristics with extended T(1/2) from 9.4 to 14 h compared to conventional formulation. Anti-inflammatory activity of BUD and developed formulations was compared and found to be similar. Cytotoxicity was determined in A549 alveolar epithelial cell line and found to be not toxic. In vivo pulmonary deposition of developed conventional formulation was studied using gamma scintigraphy and results indicated potential in vitro-in vivo correlation in performance of conventional BUD DPI formulation. From the DPI formulation prepared with porous particles, the concentration of BUD increased fourfold in the lungs, indicating pulmonary targeting potential of developed formulations.
Figures



Similar articles
-
Rehydrated sterically stabilized phospholipid nanomicelles of budesonide for nebulization: physicochemical characterization and in vitro, in vivo evaluations.Int J Nanomedicine. 2011;6:2351-66. doi: 10.2147/IJN.S25363. Epub 2011 Oct 14. Int J Nanomedicine. 2011. PMID: 22072872 Free PMC article.
-
Design and evaluation of novel inhalable sildenafil citrate spray-dried microparticles for pulmonary arterial hypertension.J Control Release. 2019 May 28;302:126-139. doi: 10.1016/j.jconrel.2019.03.029. Epub 2019 Mar 30. J Control Release. 2019. PMID: 30940497
-
Inhalable co-amorphous budesonide-arginine dry powders prepared by spray drying.Int J Pharm. 2019 Jun 30;565:1-8. doi: 10.1016/j.ijpharm.2019.04.036. Epub 2019 Apr 15. Int J Pharm. 2019. PMID: 30999050
-
Supercritical Fluid Particle Design of DPI Formulations (Review).Curr Pharm Des. 2015;21(19):2516-42. doi: 10.2174/1381612821666150416100201. Curr Pharm Des. 2015. PMID: 25876911 Review.
-
Modern trends in the formulation of microparticles for lung delivery using porogens: methods, principles and examples.Pharm Dev Technol. 2024 Jun;29(5):504-516. doi: 10.1080/10837450.2024.2350530. Epub 2024 May 23. Pharm Dev Technol. 2024. PMID: 38712608 Review.
Cited by
-
Pulmonary route of administration is instrumental in developing therapeutic interventions against respiratory diseases.Saudi Pharm J. 2020 Dec;28(12):1655-1665. doi: 10.1016/j.jsps.2020.10.012. Epub 2020 Nov 4. Saudi Pharm J. 2020. Retraction in: Saudi Pharm J. 2022 May;30(5):646. doi: 10.1016/j.jsps.2022.04.011. PMID: 33424258 Free PMC article. Retracted. Review.
-
Development of Budesonide Loaded Biopolymer Based Dry Powder Inhaler: Optimization, In Vitro Deposition, and Cytotoxicity Study.J Pharm (Cairo). 2014;2014:795371. doi: 10.1155/2014/795371. Epub 2014 Jun 15. J Pharm (Cairo). 2014. PMID: 26556201 Free PMC article.
-
Therapeutic liposomal dry powder inhalation aerosols for targeted lung delivery.Lung. 2012 Jun;190(3):251-62. doi: 10.1007/s00408-011-9360-x. Epub 2012 Jan 25. Lung. 2012. PMID: 22274758 Review.
-
Development and evaluation of inhalational liposomal system of budesonide for better management of asthma.Indian J Pharm Sci. 2010 Jul;72(4):442-8. doi: 10.4103/0250-474X.73916. Indian J Pharm Sci. 2010. PMID: 21218054 Free PMC article.
-
Preparation and Characterization of a Dry Powder Inhaler Composed of PLGA Large Porous Particles Encapsulating Gentamicin Sulfate.Adv Pharm Bull. 2019 Jun;9(2):255-261. doi: 10.15171/apb.2019.029. Epub 2019 Jun 1. Adv Pharm Bull. 2019. PMID: 31380251 Free PMC article.
References
-
- Basyigit I, Yildiz F, Ozkara SK, Yildirim E, Boyaci H, Ilgazli A. Addition of inhaled corticosteroid on combined bronchodilator therapy in patients with COPD. Pulm Pharmacol Ther. 2005;20:1–5. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

