Enantioselective total synthesis of brevetoxin A: unified strategy for the B, E, G, and J subunits
- PMID: 19650091
- PMCID: PMC2826130
- DOI: 10.1002/chem.200900776
Enantioselective total synthesis of brevetoxin A: unified strategy for the B, E, G, and J subunits
Abstract
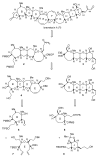
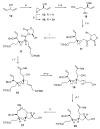
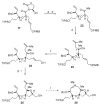
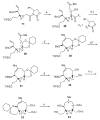
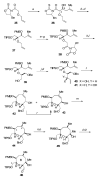
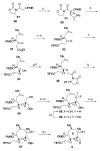
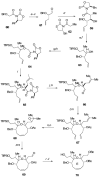
Brevetoxin A is a decacyclic ladder toxin that possesses 5-, 6-, 7-, 8-, and 9-membered oxacycles, as well as 22 tetrahedral stereocenters. Herein, we describe a unified approach to the B, E, G, and J rings based upon a ring-closing metathesis strategy from the corresponding dienes. The enolate technologies developed in our laboratory allowed access to the precursor acyclic dienes for the B, E, and G medium-ring ethers. The strategies developed for the syntheses of these four monocycles ultimately provided multigram quantities of each of the rings, supporting our efforts toward the completion of a convergent synthesis of brevetoxin A.
Figures

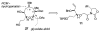

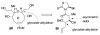
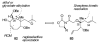
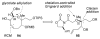
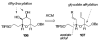

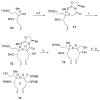
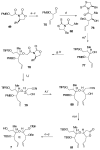
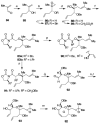
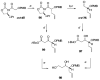

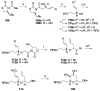
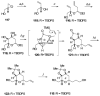
References
-
- Nicolaou KC, Frederick MO, Aversa RJ. Angew Chem Int Ed. 2008;47:7182. - PMC - PubMed
- Fuwa H, Sasaki M. Curr Opin Drug Discovery Dev. 2007;10:784. - PubMed
- Inoue M. Chem Rev. 2005;105:4379. - PubMed
- Nakata I. Chem Rev. 2005;105:4314. - PubMed
- Kadota I, Yamamoto Y. Acc Chem Res. 2005;38:423. - PubMed
- Sasaki M, Fuwa H. Synlett. 2004;11:1851.
- Evans PA, Delouvrié B. Curr Opin Drug Discovery Dev. 2002;5:986. - PubMed
- Marmsäter FP, West FG. Chem Eur J. 2002;8:4346. - PubMed
- Mori Y. Chem Eur J. 1997;3:849.
- Alvarez E, Candenas ML, Pérez R, Ravelo JL, Martín JD. Chem Rev. 1995;95:1953.
-
- Shimizu Y, Chou HN, Bando H, Vanduyne G, Clardy JC. J Am Chem Soc. 1986;108:514. - PubMed
- Shimizu Y, Chou HN, Bando H, Van Duyne G, Clardy JC. J Chem Soc, Chem Commun. 1986:1656. - PubMed
- Pawlak J, Tempesta MS, Golik J, Zagorski MG, Lee MS, Nakanishi K, Iwashita T, Gross ML, Tomer KB. J Am Chem Soc. 1987;109:1144.
-
- Michelliza S, Abraham WM, Jacocks HM, Schuster T, Baden DG. ChemBioChem. 2007;8:2233. - PMC - PubMed
- Naar JP, Flewelling LJ, Lenzi A, Abbott JP, Granholm A, Jacocks HM, Gannon D, Henry M, Pierce R, Baden DG, Wolny J, Landsberg JH. Toxicon. 2007;50:707. - PMC - PubMed
- Kirkpatrick B, Fleming LE, Backer LC, Bean JA, Tamer R, Kirkpatrick G, Kane T, Wanner A, Dalpra D, Reich A, Baden DG. Harmful Algae. 2006;5:526. - PMC - PubMed
- Fleming LE, Backer LC, Baden DG. Environ Health Persp. 2005;113:618. and references therein. - PMC - PubMed
-
- Nicolaou KC, Yang Z, Shi GQ, Gunzner JL, Agrios KA, Gärtner P. Nature. 1998;392:264. - PubMed
- Nicolaou KC, Bunnage ME, McGarry DG, Shi S, Somers PK, Wallace PA, Chu XJ, Agrios KA, Gunzner JL, Yang Z. Chem Eur J. 1999;5:599.
- Nicolaou KC, Wallace PA, Shi S, Ouellette MA, Bunnage ME, Gunzner JL, Agrios KA, Shi Gq, Gärtner P, Yang Z. Chem Eur J. 1999;5:618.
- Nicolaou KC, Shi Gq, Gunzner JL, Gärtner P, Wallace PA, Ouellette MA, Shi S, Bunnage ME, Agrios KA, Veale CA, Hwang CK, Hutchinson J, Prasad CVC, Ogilvie WW, Yang Z. Chem Eur J. 1999;5:628.
- Nicolaou KC, Gunzner JL, Shi Gq, Agrios KA, Gärtner P, Yang Z. Chem Eur J. 1999;5:646. - PubMed
-
- Horner L, Hoffmann H, Wippel HG, Klahre G. Chem Ber. 1959;92:2499.
- Buss AD, Warren S. J Chem Soc Perkin Trans 1. 1985:2307. and references therein.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

