Thymic involution, a co-morbidity factor in amyotrophic lateral sclerosis
- PMID: 19650830
- PMCID: PMC3823164
- DOI: 10.1111/j.1582-4934.2009.00863.x
Thymic involution, a co-morbidity factor in amyotrophic lateral sclerosis
Abstract
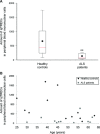
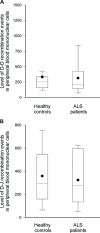
Amyotrophic lateral sclerosis (ALS) is a devastating disease, characterized by extremely rapid loss of motor neurons. Our studies over the last decade have established CD4(+) T cells as important players in central nervous system maintenance and repair. Those results, together with recent findings that CD4(+) T cells play a protective role in mouse models of ALS, led us to the current hypothesis that in ALS, a rapid T-cell malfunction may develop in parallel to the motor neuron dysfunction. Here, we tested this hypothesis by assessing thymic function, which serves as a measure of peripheral T-cell availability, in an animal model of ALS (mSOD1 [superoxide dismutase] mice; G93A) and in human patients. We found a significant reduction in thymic progenitor-cell content, and abnormal thymic histology in 3-4-month-old mSOD1 mice. In ALS patients, we found a decline in thymic output, manifested in the reduction in blood levels of T-cell receptor rearrangement excision circles, a non-invasive measure of thymic function, and demonstrated a restricted T-cell repertoire. The morbidity of the peripheral immune cells was also manifested in the increase of pro-apoptotic BAX/BCXL2 expression ratio in peripheral blood mononuclear cells (PBMCs) of these patients. In addition, gene expression screening in the same PBMCs, revealed in the ALS patients a reduction in key genes known to be associated with T-cell activity, including: CD80, CD86, IFNG and IL18. In light of the reported beneficial role of T cells in animal models of ALS, the present observation of thymic dysfunction, both in human patients and in an animal model, might be a co-pathological factor in ALS, regardless of the disease aetiology. These findings may lead to the development of novel therapeutic approaches directed at overcoming the thymic defect and T-cell deficiency.
© 2009 The Authors Journal compilation © 2010 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures



References
-
- Lee JR, Annegers JF, Appel SH. Prognosis of amyotrophic lateral sclerosis and the effect of referral selection. J Neurol Sci. 1995;132:207–15. - PubMed
-
- Rosen DR. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. - PubMed
-
- Boillee S, Yamanaka K, Lobsiger CS, et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–92. - PubMed
-
- Clement AM, Nguyen MD, Roberts EA, et al. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003;302:113–7. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

