X-ray diffraction and reflectivity validation of the depletion attraction in the competitive adsorption of lung surfactant and albumin
- PMID: 19651036
- PMCID: PMC2718144
- DOI: 10.1016/j.bpj.2009.05.017
X-ray diffraction and reflectivity validation of the depletion attraction in the competitive adsorption of lung surfactant and albumin
Abstract
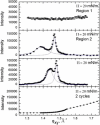
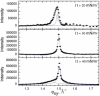
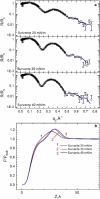
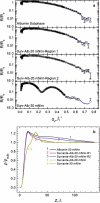
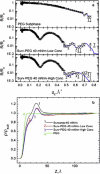
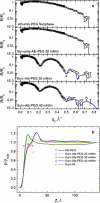
Lung surfactant (LS) and albumin compete for the air-water interface when both are present in solution. Equilibrium favors LS because it has a lower equilibrium surface pressure, but the smaller albumin is kinetically favored by faster diffusion. Albumin at the interface creates an energy barrier to subsequent LS adsorption that can be overcome by the depletion attraction induced by polyethylene glycol (PEG) in solution. A combination of grazing incidence x-ray diffraction (GIXD), x-ray reflectivity (XR), and pressure-area isotherms provides molecular-resolution information on the location and configuration of LS, albumin, and polymer. XR shows an average electron density similar to that of albumin at low surface pressures, whereas GIXD shows a heterogeneous interface with coexisting LS and albumin domains at higher surface pressures. Albumin induces a slightly larger lattice spacing and greater molecular tilt, similar in effect to a small decrease in the surface pressure. XR shows that adding PEG to the LS-albumin subphase restores the characteristic LS electron density profile at the interface, and confirms that PEG is depleted near the interface. GIXD shows the same LS Bragg peaks and Bragg rods as on a pristine interface, but with a more compact lattice corresponding to a small increase in the surface pressure. These results confirm that albumin adsorption creates a physical barrier that inhibits LS adsorption, and that PEG in the subphase generates a depletion attraction between the LS aggregates and the interface that enhances LS adsorption without substantially altering the structure or properties of the LS monolayer.
Figures


References
-
- Marsh D. Lateral pressure in membranes. Biochim. Biophys. Acta. 1996;1286:183–223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

