Modulation of titin-based stiffness by disulfide bonding in the cardiac titin N2-B unique sequence
- PMID: 19651040
- PMCID: PMC2718153
- DOI: 10.1016/j.bpj.2009.05.037
Modulation of titin-based stiffness by disulfide bonding in the cardiac titin N2-B unique sequence
Abstract
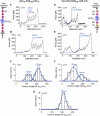
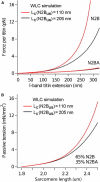
The giant protein titin is responsible for the elasticity of nonactivated muscle sarcomeres. Titin-based passive stiffness in myocardium is modulated by titin-isoform switching and protein-kinase (PK)A- or PKG-dependent titin phosphorylation. Additional modulatory effects on titin stiffness may arise from disulfide bonding under oxidant stress, as many immunoglobulin-like (Ig-)domains in titin's spring region have a potential for S-S formation. Using single-molecule atomic force microscopy (AFM) force-extension measurements on recombinant Ig-domain polyprotein constructs, we show that titin Ig-modules contain no stabilizing disulfide bridge, contrary to previous belief. However, we demonstrate that the human N2-B-unique sequence (N2-B(us)), a cardiac-specific, physiologically extensible titin segment comprising 572 amino-acid residues, contains up to three disulfide bridges under oxidizing conditions. AFM force spectroscopy on recombinant N2-B(us) molecules demonstrated a much shorter contour length in the absence of a reducing agent than in its presence, consistent with intramolecular S-S bonding. In stretch experiments on isolated human heart myofibrils, the reducing agent thioredoxin lowered titin-based stiffness to a degree that could be explained (using entropic elasticity theory) by altered extensibility solely of the N2-B(us). We conclude that increased oxidant stress can elevate titin-based stiffness of cardiomyocytes, which may contribute to the global myocardial stiffening frequently seen in the aging or failing heart.
Figures



References
-
- Granzier H., Labeit S. Structure-function relations of the giant elastic protein titin in striated and smooth muscle cells. Muscle Nerve. 2007;36:740–755. - PubMed
-
- Linke W.A. Sense and stretchability: the role of titin and titin-associated proteins in myocardial stress-sensing and mechanical dysfunction. Cardiovasc. Res. 2008;77:637–648. - PubMed
-
- Neagoe C., Kulke M., del Monte F., Gwathmey J.K., de Tombe P.P. Titin isoform switch in ischemic human heart disease. Circulation. 2002;106:1333–1341. - PubMed
-
- Nagueh S.F., Shah G., Wu Y., Torre-Amione G., King N.M. Altered titin expression, myocardial stiffness, and left ventricular function in patients with dilated cardiomyopathy. Circulation. 2004;110:155–162. - PubMed
-
- Makarenko I., Opitz C.A., Leake M.C., Neagoe C., Kulke M. Passive stiffness changes caused by upregulation of compliant titin isoforms in human DCM hearts. Circ. Res. 2004;95:708–716. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

