Structural and functional architecture of respiratory networks in the mammalian brainstem
- PMID: 19651658
- PMCID: PMC2865112
- DOI: 10.1098/rstb.2009.0081
Structural and functional architecture of respiratory networks in the mammalian brainstem
Abstract
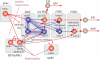
Neural circuits controlling breathing in mammals are organized within serially arrayed and functionally interacting brainstem compartments extending from the pons to the lower medulla. The core circuit components that constitute the neural machinery for generating respiratory rhythm and shaping inspiratory and expiratory motor patterns are distributed among three adjacent structural compartments in the ventrolateral medulla: the Bötzinger complex (BötC), pre-Bötzinger complex (pre-BötC) and rostral ventral respiratory group (rVRG). The respiratory rhythm and inspiratory-expiratory patterns emerge from dynamic interactions between: (i) excitatory neuron populations in the pre-BötC and rVRG active during inspiration that form inspiratory motor output; (ii) inhibitory neuron populations in the pre-BötC that provide inspiratory inhibition within the network; and (iii) inhibitory populations in the BötC active during expiration that generate expiratory inhibition. Network interactions within these compartments along with intrinsic rhythmogenic properties of pre-BötC neurons form a hierarchy of multiple oscillatory mechanisms. The functional expression of these mechanisms is controlled by multiple drives from more rostral brainstem components, including the retrotrapezoid nucleus and pons, which regulate the dynamic behaviour of the core circuitry. The emerging view is that the brainstem respiratory network has rhythmogenic capabilities at multiple hierarchical levels, which allows flexible, state-dependent expression of different rhythmogenic mechanisms under different physiological and metabolic conditions and enables a wide repertoire of respiratory behaviours.
Figures



References
-
- Alheid G. F., McCrimmon D. R.2008The chemical neuroanatomy of breathing. Respir. Physiol. Neurobiol. 164, 3–11 (doi:10.1016/j.resp.2008.07.014) - DOI - PMC - PubMed
-
- Alheid G. F., Milsom W. K., McCrimmon D. R.2004Pontine influences on breathing: an overview. Respir. Physiol. Neurobiol. 143, 105–114 (doi:10.1016/j.resp.2004.06.016) - DOI - PubMed
-
- Bianchi A. L., Denavit-Saubie M., Champagnat J.1995Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol. Rev. 75, 1–45 - PubMed
-
- Butera R. J., Rinzel J. R., Smith J. C.1999aModels of respiratory rhythm generation in the pre-Bötzinger complex. I. Bursting pacemaker neurons. J. Neurophysiol. 82, 382–397 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

