Wnt-5a-induced phosphorylation of DARPP-32 inhibits breast cancer cell migration in a CREB-dependent manner
- PMID: 19651774
- PMCID: PMC2785682
- DOI: 10.1074/jbc.M109.048884
Wnt-5a-induced phosphorylation of DARPP-32 inhibits breast cancer cell migration in a CREB-dependent manner
Abstract
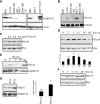
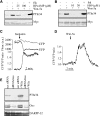
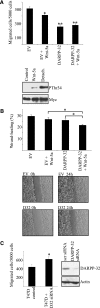
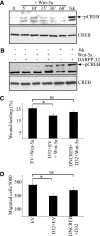
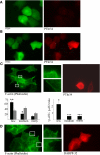
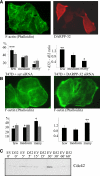
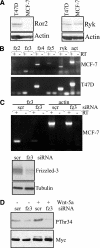
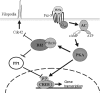
Tumor cell migration plays a central role in the process of cancer metastasis. We recently identified dopamine and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32) as an antimigratory phosphoprotein in breast cancer cells. Here we link this effect of DARPP-32 to Wnt-5a signaling by demonstrating that recombinant Wnt-5a triggers cAMP elevation at the plasma membrane and Thr34-DARPP-32 phosphorylation in MCF-7 cells. In agreement, both protein kinase A (PKA) inhibitors and siRNA-mediated knockdown of Frizzled-3 receptor or Galpha(s) expression abolished Wnt-5a-induced phosphorylation of DARPP-32. Furthermore, Wnt-5a induced DARPP-32-dependent inhibition of MCF-7 cell migration. Phospho-Thr-34-DARPP-32 interacted with protein phosphatase-1 (PP1) and potentiated the Wnt-5a-mediated phosphorylation of CREB, a well-known PP1 substrate, but had no effect on CREB phosphorylation by itself. Moreover, inhibition of the Wnt-5a/DARPP-32/CREB pathway, by expression of dominant negative CREB (DN-CREB), diminished the antimigratory effect of Wnt-5a-induced phospho-Thr-34-DARPP-32. Phalloidin-staining revealed that that the presence of phospho-Thr-34-DARPP-32 in MCF-7 cells results in reduced filopodia formation. In accordance, the activity of the Rho GTPase Cdc42, known to be crucial for filopodia formation, was reduced in MCF-7 cells expressing phospho-Thr-34-DARPP-32. The effects of DARPP-32 on cell migration and filopodia formation could be reversed in T47D breast cancer cells that were depleted of their endogenous DARPP-32 by siRNA targeting. Consequently, Wnt-5a activates a Frizzled-3/Galpha(s)/cAMP/PKA signaling pathway that triggers a DARPP-32- and CREB-dependent antimigratory response in breast cancer cells, representing a novel mechanism whereby Wnt-5a can inhibit breast cancer cell migration.
Figures

References
-
- Hansen C., Greengard P., Nairn A. C., Andersson T., Vogel W. F. (2006) Exp. Cell Res. 312, 4011–4018 - PubMed
-
- Walaas S. I., Aswad D. W., Greengard P. (1983) Nature 301, 69–71 - PubMed
-
- Hemmings H. C., Jr., Greengard P., Tung H. Y., Cohen P. (1984) Nature 310, 503–505 - PubMed
-
- Bibb J. A., Snyder G. L., Nishi A., Yan Z., Meijer L., Fienberg A. A., Tsai L. H., Kwon Y. T., Girault J. A., Czernik A. J., Huganir R. L., Hemmings H. C., Jr., Nairn A. C., Greengard P. (1999) Nature 402, 669–671 - PubMed
-
- Svenningsson P., Nishi A., Fisone G., Girault J. A., Nairn A. C., Greengard P. (2004) Annu. Rev. Pharmacol. Toxicol. 44, 269–296 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

