Rapamycin rescues TDP-43 mislocalization and the associated low molecular mass neurofilament instability
- PMID: 19651785
- PMCID: PMC2785671
- DOI: 10.1074/jbc.M109.031278
Rapamycin rescues TDP-43 mislocalization and the associated low molecular mass neurofilament instability
Abstract
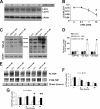
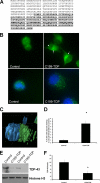
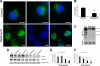
TDP-43 is a nuclear protein involved in exon skipping and alternative splicing. Recently, TDP-43 has been identified as the pathological signature protein in frontotemporal lobar degeneration with ubiquitin-positive inclusions and in amyotrophic lateral sclerosis. In addition, TDP-43-positive inclusions are present in Parkinson disease, dementia with Lewy bodies, and 30% of Alzheimer disease cases. Pathological TDP-43 is redistributed from the nucleus to the cytoplasm, where it accumulates. An approximately 25-kDa C-terminal fragment of TDP-43 accumulates in affected brain regions, suggesting that it may be involved in the disease pathogenesis. Here, we show that overexpression of the 25-kDa C-terminal fragment is sufficient to cause the mislocalization and cytoplasmic accumulation of endogenous full-length TDP-43 in two different cell lines, thus recapitulating a key biochemical characteristic of TDP-43 proteinopathies. We also found that TDP-43 mislocalization is associated with a reduction in the low molecular mass neurofilament mRNA levels. Notably, we show that the autophagic system plays a role in TDP-43 metabolism. Specifically, we found that autophagy inhibition increases the accumulation of the C-terminal fragments of TDP-43, whereas inhibition of mTOR, a key protein kinase involved in autophagy regulation, reduces the 25-kDa C-terminal fragment accumulation and restores TDP-43 localization. Our results suggest that autophagy induction may be a valid therapeutic target for TDP-43 proteinopathies.
Figures
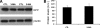


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

