Dystrophin is a microtubule-associated protein
- PMID: 19651889
- PMCID: PMC2728405
- DOI: 10.1083/jcb.200905048
Dystrophin is a microtubule-associated protein
Abstract
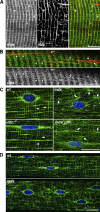
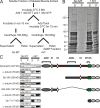
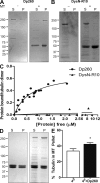
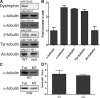
Cytolinkers are giant proteins that can stabilize cells by linking actin filaments, intermediate filaments, and microtubules (MTs) to transmembrane complexes. Dystrophin is functionally similar to cytolinkers, as it links the multiple components of the cellular cytoskeleton to the transmembrane dystroglycan complex. Although no direct link between dystrophin and MTs has been documented, costamere-associated MTs are disrupted when dystrophin is absent. Using tissue-based cosedimentation assays on mice expressing endogenous dystrophin or truncated transgene products, we find that constructs harboring spectrinlike repeat 24 through the first third of the WW domain cosediment with MTs. Purified Dp260, a truncated isoform of dystrophin, bound MTs with a K(d) of 0.66 microM, a stoichiometry of 1 Dp260/1.4 tubulin heterodimer at saturation, and stabilizes MTs from cold-induced depolymerization. Finally, alpha- and beta-tubulin expression is increased approximately 2.5-fold in mdx skeletal muscle without altering the tubulin-MT equilibrium. Collectively, these data suggest dystrophin directly organizes and/or stabilizes costameric MTs and classifies dystrophin as a cytolinker in skeletal muscle.
Figures

References
-
- Altman A., Szyper-Kravitz M., Shoenfeld Y. 2007. Colchicine-induced rhabdomyolysis.Clin. Rheumatol. 26:2197–2199 - PubMed
-
- Ayalon G., Davis J.Q., Scotland P.B., Bennett V. 2008. An ankyrin-based mechanism for functional organization of dystrophin and dystroglycan.Cell. 135:1189–1200 - PubMed
-
- Bhosle R.C., Michele D.E., Campbell K.P., Li Z., Robson R.M. 2006. Interactions of intermediate filament protein synemin with dystrophin and utrophin.Biochem. Biophys. Res. Commun. 346:768–777 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

