A role of histone H3 lysine 4 methyltransferase components in endosomal trafficking
- PMID: 19651892
- PMCID: PMC2728403
- DOI: 10.1083/jcb.200902146
A role of histone H3 lysine 4 methyltransferase components in endosomal trafficking
Abstract
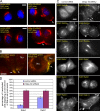
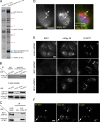
Histone lysine methyltransferase complexes are essential for chromatin organization and gene regulation. Whether any of this machinery functions in membrane traffic is unknown. In this study, we report that mammal Dpy-30 (mDpy-30), a subunit of several histone H3 lysine 4 (H3K4) methyltransferase (H3K4MT) complexes, resides in the nucleus and at the trans-Golgi network (TGN). The TGN targeting of mDpy-30 is mediated by BIG1, a TGN-localized guanine nucleotide exchange factor for adenosine diphosphate ribosylation factor GTPases. Altering mDpy-30 levels changes the distribution of cation-independent mannose 6-phosphate receptor (CIMPR) without affecting that of TGN46 or transferrin receptor. Our experiments also indicate that mDpy-30 functions in the endosome to TGN transport of CIMPR and that its knockdown results in the enrichment of internalized CIMPR and recycling endosomes near cell protrusions. Much like mDpy-30 depletion, the knockdown of Ash2L or RbBP5, two other H3K4MT subunits, leads to a similar redistribution of CIMPR. Collectively, these results suggest that mDpy-30 and probably H3K4MT play a role in the endosomal transport of specific cargo proteins.
Figures



References
-
- Cai H., Reinisch K., Ferro-Novick S. 2007. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev. Cell. 12:671–682. - PubMed
-
- Caswell P.T., Norman J.C. 2006. Integrin trafficking and the control of cell migration. Traffic. 7:14–21. - PubMed
-
- Caswell P., Norman J. 2008. Endocytic transport of integrins during cell migration and invasion. Trends Cell Biol. 18:257–263. - PubMed
-
- Chapman H.A. 1997. Plasminogen activators, integrins, and the coordinated regulation of cell adhesion and migration. Curr. Opin. Cell Biol. 9:714–724. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

