Drug-sensitized zebrafish screen identifies multiple genes, including GINS3, as regulators of myocardial repolarization
- PMID: 19652097
- PMCID: PMC2771327
- DOI: 10.1161/CIRCULATIONAHA.108.821082
Drug-sensitized zebrafish screen identifies multiple genes, including GINS3, as regulators of myocardial repolarization
Abstract
Background: Cardiac repolarization, the process by which cardiomyocytes return to their resting potential after each beat, is a highly regulated process that is critical for heart rhythm stability. Perturbations of cardiac repolarization increase the risk for life-threatening arrhythmias and sudden cardiac death. Although genetic studies of familial long-QT syndromes have uncovered several key genes in cardiac repolarization, the major heritable contribution to this trait remains unexplained. Identification of additional genes may lead to a better understanding of the underlying biology, aid in identification of patients at risk for sudden death, and potentially enable new treatments for susceptible individuals.
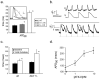
Methods and results: We extended and refined a zebrafish model of cardiac repolarization by using fluorescent reporters of transmembrane potential. We then conducted a drug-sensitized genetic screen in zebrafish, identifying 15 genes, including GINS3, that affect cardiac repolarization. Testing these genes for human relevance in 2 concurrently completed genome-wide association studies revealed that the human GINS3 ortholog is located in the 16q21 locus, which is strongly associated with QT interval.
Conclusions: This sensitized zebrafish screen identified 15 novel myocardial repolarization genes. Among these genes is GINS3, the human ortholog of which is a major locus in 2 concurrent human genome-wide association studies of QT interval. These results reveal a novel network of genes that regulate cardiac repolarization.
Figures


References
-
- Priori SG, Napolitano C, Schwartz PJ, Grillo M, Bloise R, Ronchetti E, Moncalvo C, Tulipani C, Veia A, Bottelli G, Nastoli J. Association of long QT syndrome loci and cardiac events among patients treated with beta-blockers. Jama. 2004;292:1341–4. - PubMed
-
- Siscovick DS, Raghunathan TE, Rautaharju P, Psaty BM, Cobb LA, Wagner EH. Clinically silent electrocardiographic abnormalities and risk of primary cardiac arrest among hypertensive patients. Circulation. 1996;94:1329–33. - PubMed
-
- Brooksby P, Batin PD, Nolan J, Lindsay SJ, Andrews R, Mullen M, Baig W, Flapan AD, Prescott RJ, Neilson JM, Cowley AJ, Fox KA. The relationship between QT intervals and mortality in ambulant patients with chronic heart failure. The united kingdom heart failure evaluation and assessment of risk trial (UK-HEART) Eur Heart J. 1999;20:1335–41. - PubMed
-
- Roden DM. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–22. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

