Toward clinical therapies using hematopoietic cells derived from human pluripotent stem cells
- PMID: 19652198
- PMCID: PMC2766673
- DOI: 10.1182/blood-2009-03-191304
Toward clinical therapies using hematopoietic cells derived from human pluripotent stem cells
Abstract
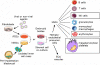
Human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) provide remarkable cellular platforms to better understand human hematopoiesis and to develop clinically applicable hematopoietic cell-based therapies. Over the past decade, hESCs have been used to characterize molecular and cellular mechanisms underpinning the differentiation of hematopoietic progenitors and mature, functional hematopoietic cells. These advances are now poised to lead to clinical translation of hESC- and iPSC-derived hematopoietic cells for novel therapies in the next few years. On the basis of areas of recent success, initial clinical use of hematopoietic cells derived from human pluripotent stem cells will probably be in the areas of transfusion therapies (erythrocytes and platelets) and immune therapies (natural killer cells). In contrast, efficient development and isolation of hematopoietic stem cells capable of long-term, multilineage engraftment still remains a significant challenge. Technical, safety, and regulatory concerns related to clinical applications of human PSCs must be appropriately addressed. However, proper consideration of these issues should facilitate and not inhibit clinical translation of new therapies. This review outlines the current status of hematopoietic cell development and what obstacles must be surmounted to bring hematopoietic cell therapies from human PSCs from "bench to bedside."
Figures
References
-
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–1147. - PubMed
-
- Kyba M, Daley GQ. Hematopoiesis from embryonic stem cells: lessons from and for ontogeny. Exp Hematol. 2003;31(11):994–1006. - PubMed
-
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. - PubMed
-
- Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448(7151):313–317. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

